- EN - English
- CN - 中文
Visualizing diverse RNA functions in living cells with Spinach™ family of fluorogenic aptamers
利用SpinachTM系列荧光适配体可视化活细胞中多种RNA功能
发布: 2026年05月05日第16卷第9期 DOI: 10.21769/BioProtoc.5504 浏览次数: 1233
评审: Marion HoggJose M. DiasAnonymous reviewer(s)
Abstract
RNA is now recognized as a highly diverse and dynamic class of molecules whose localization, processing, and turnover are central to cell function and disease. Live-cell RNA imaging is therefore essential for linking RNA behavior to mechanism. Existing approaches include quenched hybridization probes that directly target endogenous transcripts but face delivery and sequestration issues, protein-recruitment tags such as MS2/PP7 that add large payloads and can perturb localization or decay, and CRISPR–dCas13 imaging that requires substantial protein cargo and careful control of background and off-target effects. Here, we present a protocol for live-cell RNA imaging using the SpinachTM family of fluorogenic RNA aptamers. The method details the design and cloning of SpinachTM-tagged RNA constructs, selection and handling of cognate small-molecule fluorophores, expression in mammalian cell lines, dye loading, and image acquisition on standard fluorescence microscopes, followed by quantitative analysis of localization and dynamics. We include controls to verify aptamer expression and signal specificity, guidance for multiplexing with related variants (e.g., Broccoli, Corn, Squash, Beetroot), and troubleshooting for dye permeability and signal optimization. Application examples illustrate use in tracking cellular delivery of mRNA therapeutics, monitoring transcription and decay in response to perturbations, and the forming of toxic RNA aggregates. Compared with prior methods, SpinachTM tags are compact, genetically encodable, and fluorogenic, providing high-contrast imaging in both the nucleus and cytoplasm with single-vector simplicity and multiplexing capability. The protocol standardizes key steps to improve robustness and reproducibility across cell types and laboratories.
Key features
• This protocol demonstrates the usage of the SpinachTM technology in different RNA-focused applications.
• The protocol can be used to fluorescently image most RNA types in live cells.
• This protocol requires pre-existing experience in molecular cloning, cell culturing, and fluorescence microscopy.
• Requires at least seven days to complete from beginning to end.
Keywords: RNA imaging (RNA成像)Graphical overview
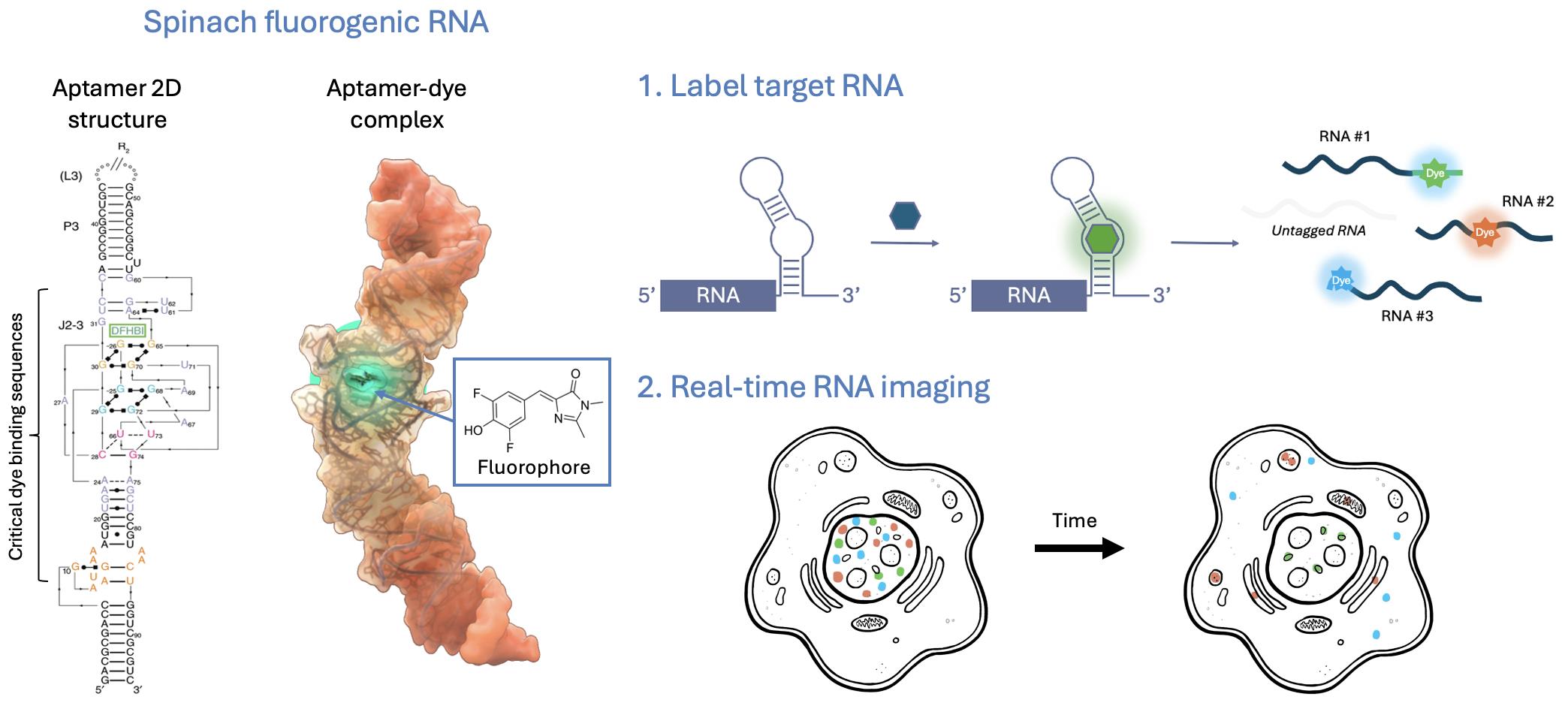
Graphical representation of the SpinachTM technology principle
Background
For decades, cellular complexity was attributed primarily to protein diversity. It is now clear that RNA populations are extraordinarily diverse, encompassing not only mRNA, rRNA, and tRNA but also numerous noncoding classes, including long noncoding RNAs (lncRNAs), microRNAs, PIWI-interacting RNAs, circular RNAs, and more [1]. Current human genome annotations list ~19–20k protein-coding genes versus >35k lncRNA genes, underscoring that annotated noncoding genes now outnumber protein-coding genes [2]. Further, thousands of small noncoding RNAs are also cataloged. Emerging work continues to define additional small-RNA subclasses, their biogenesis, and regulatory roles across physiology and disease.
RNA fate is shaped by compartmentalized processing events—capping, splicing, editing, modification, decay, and localization—many of which occur in, or are regulated by, nuclear and cytoplasmic ribonucleoprotein condensates (e.g., nucleolus, Cajal bodies, nuclear speckles, and paraspeckles) [3]. These bodies depend on RNA for integrity and function and are increasingly linked to human disease. In the nervous system, RNA localization and local translation in axons, dendrites, and synapses support development and plasticity, making spatial RNA regulation particularly consequential [4,5]. Techniques that resolve RNA dynamics in living cells are therefore essential for connecting localization and processing to function.
Genetically encodable fluorescent proteins transformed cell biology by enabling routine, live-cell visualization of protein localization and trafficking. Analogous tools for RNA have matured substantially, but each strategy carries distinct trade-offs that must be considered when selecting methods for live-cell imaging.
Quenched hybridization probes (molecular beacons) directly target endogenous transcripts and provide fluorogenic turn-on and spectral multiplexing, but face delivery and nuclear sequestration issues and require target-specific designs [6]. Protein-recruitment tags (MS2/PP7 stem-loops with coat proteins) remain a widely used approach for single-mRNA tracking and are compatible with genome editing. However, these systems add large RNA payloads, introduce background signals from unbound coat proteins, and can perturb RNA biology through altered localization or impaired stability [7,8]. CRISPR–dCas13 imaging enables programmable detection of endogenous RNAs, yet its application is limited by the large protein cargo and potential off-target effects, and can introduce fluorescence from unbound complexes [9,10].
Fluorogenic RNA aptamers (also called fluorescence light-up aptamers) are short, structured RNA sequences that bind non-fluorescent small-molecule dyes and activate their fluorescence upon binding. Aptamer-dye pairs such as SpinachTM family, Mango, and Peppers provide genetically encodable, compact tags with high signal-to-background contrast and expanding orthogonal palettes that enable powerful multiplex imaging. Compared with protein-based or probe-based approaches, fluorogenic aptamers offer smaller genetic footprints, direct integration into native transcripts, and highly versatile applications from single-molecule tracking to high-throughput assays. Although aptamer folding efficiency remains a technical concern, cellular perturbations have not been widely reported, and careful design can further minimize risks of altering RNA biology. Importantly, the high specificity of fluorophore binding arises from unique three-dimensional aptamer folding that creates well-defined binding pockets, thereby preventing interaction with unrelated structured RNAs and minimizing off-target effects [11,12]. With ongoing engineering to improve folding robustness, labeling efficiency, and dye performance, fluorogenic RNA aptamers are rapidly emerging as one of the most versatile and biologically compatible tools for live-cell RNA imaging.
This protocol provides a focused guide to the use and applications of the SpinachTM family of fluorogenic RNA aptamers. Spinach was among the first genetically encodable RNA aptamers to enable robust fluorogenic imaging in living cells [13]. Here, we use “SpinachTM family” as an umbrella term for Spinach and related improved aptamers (e.g., Broccoli, Corn, Squash, Beetroot), together with their cognate small-molecule fluorogens (e.g., DFHBI, DFHBI-1T, DFHO, BI). These fluorophores are cell-permeable and, when bound by their matching aptamers, become highly fluorescent with minimal background in cells. In the absence of the aptamer, they exhibit little to no fluorescence, enabling high contrast. As genetically encodable tags, SpinachTM family aptamers have been widely used to interrogate RNA localization, dynamics, and regulation—both from exogenous constructs and at endogenous loci—across diverse biological and disease contexts [14–22].
Members of the SpinachTM aptamer family are named for vegetables whose colors mirror their emission (e.g., Spinach/Broccoli: green; Corn: yellow; Squash: orange; Beetroot/Red Broccoli: red). This convention echoes the color-themed, fruit-inspired nomenclature popularized for fluorescent protein variants.
In this protocol, we describe step-by-step how to generate SpinachTM-tagged RNA constructs and express them in mammalian cell lines, acquire live-cell imaging, and analyze the resulting data. We also present selected application examples that highlight how these tools can be deployed across varied cellular applications.
Materials and reagents
Biological materials
1. HeLa human cervical epithelial cells (ATCC, catalog number: CRM-CRL-2)
2. HEK293T human kidney epithelial cells (ATCC, catalog number: CRL-3216)
3. COS-7 monkey fibroblasts (ATCC, catalog number: CRL-1651)
Reagents
1. DMEM, high glucose, GlutaMaxTM, pyruvate, phenol red (ThermoFisher, catalog number: 10569010)
2. Fetal bovine serum (FBS) (ThermoFisher, catalog number: 26140079)
3. Penicillin-streptomycin solution (pen-strep) (ThermoFisher, catalog number: 15140122)
4. TrypLETM, no phenol red (ThermoFisher, catalog number: 12563011)
5. GlutaMaxTM supplement (ThermoFisher, catalog number: 35050061)
6. Cell culture-grade water (Millipore Sigma, catalog number: W3500-6X500ML)
7. Phosphate-buffered saline, 1× (PBS) (Corning, catalog number: 21-040-CV)
8. Trypan Blue, 0.4% solution (Thomas Scientific, catalog number: C838W29)
9. Poly-L-lysine solution, 0.1 mg/mL (PLL) (Millipore Sigma, catalog number: A-005-C)
10. Human plasma fibronectin, 5 mg (Corning, catalog number: 356008)
11. FuGENE® HD (Promega, catalog number: E2311)
12. Opti-MEM (ThermoFisher, catalog number: 31985062)
13. FluoroBriteTM DMEM (ThermoFisher, catalog number: A1896702)
14. Hoechst 33342, 10 mg/mL (ThermoFisher, catalog number: H3570)
15. SYTOXTM red dead cell stain, 5 μM (ThermoFisher, catalog number: S34859)
16. Plasmids:
a. pcDNA3.1-mCherry-48xBroccoli-polyA150 (generated in-house)
b. pAV-U6-Corn (Addgene, catalog number: 106233)
c. pAV-U6-F30-2xdBroccoli (Addgene, catalog number: 66842)
d. pAV-U6-Squash (Addgene, catalog number: 177913)
e. pcDNA3.1-MAPT-4xSquash (generated in-house)
f. pcDNA3.1-CUG12-2xSquash (generated in-house)
g. pcDNA3.1-CUG240-2xSquash (generated in-house)
h. pcDNA3.1-CGG60-Spinach2 (generated in-house)
i. pEGFP (Addgene, catalog number: 165830)
17. BI fluorophore (Lucerna, catalog number: 600-1mg)
18. DFHO fluorophore (Lucerna, catalog number: 500-1mg)
19. Dimethyl sulfoxide, cell culture grade (DMSO) (VWR, catalog number: IC0219605580)
20. Nikon Immersion Oil Type F, N = 1.516 (Morrell Instrument Co., catalog number: MXA22168)
21. HiScribe® T7ARCA mRNA kit (NEB, catalog number: E2060S)
22. RNA clean & concentrator (Zymo, catalog number: R1018)
Solutions
1. Cell culture media (see Recipes)
2. Fibronectin stock solution (see Recipes)
3. BI fluorophore stock solution (see Recipes)
4. DFHO fluorophore stock solution (see Recipes)
5. 1× live-cell imaging solution (see Recipes)
Recipes
1. Cell culture media
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| DMEM | 89% | 445 mL |
| FBS | 10% | 50 mL |
| Pen-strep | 1% | 5 mL |
| Total | 500 mL |
2. Fibronectin stock solution
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Human fibronectin | 1 mg/mL | 5 mg |
| Sterile ddH2O | 5 mL | |
| Total | 5 mL |
Note: Store fibronectin stock solutions in 50 μL aliquots at -80 °C. Avoid multiple freeze-thaw cycles.
3. BI fluorophore stock solution
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| BI fluorophore (M.W. 368.3) | 40 mM | 1 mg |
| DMSO | 68 μL |
Note: Store BI stock solutions at -20 °C in the dark.
4. DFHO fluorophore stock solution
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| DFHO fluorophore (M.W. 281.2) | 40 mM | 1 mg |
| DMSO | 90 μL |
Note: Store DFHO stock solutions at -20 °C in the dark.
5. 1× live-cell imaging media
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| FluoroBrite DMEM | 1× | 499.4 μL |
| Hoechst 33342, 10 mg/mL | 5 μg/mL | 0.25 μL |
| SYTOX red dead cell stain, 5 μM | 2.5 nM | 0.25 μL |
| Fluorophore stock solution*, 40 mM | 10 μM | 0.125 μL |
| Total | 500 μL/well |
Note: 0.5 mL is sufficient for one well. Determine the total volume needed by multiplying the total number of wells needed by 0.5 mL and adding a 10% excess.
*Fluorophore(s) selection should correspond to the SpinachTM tag(s) used (see Table 1). If multiplexing, two spectrally separated fluorophores should be used (e.g., Broccoli and Squash).
Table 1. Properties of SpinachTM tags
| SpinachTM tag | Length (nt) | Fluorophore | Ex (nm) | Em (nm) | Kd (nM) | Note |
| Broccoli | 49 | BI | 470 | 505 | 51 | Multiplex w/ squash, photostable |
| Spinach2 | 95 | DFHBI-1T | 482 | 505 | 560 | Ideal for bacterial imaging |
| Broccoli | 49 | DFHBI-1T | 472 | 507 | 305 | Bacterial, in vitro, cell imaging |
| Corn | 28 | DFHO | 505 | 545 | 70 | Forms dimer, can induce condensate |
| Squash | 71 | DFHO | 495 | 562 | 58 | Multiplex w/ broccoli, photostable |
| Red Broccoli | 54 | OBI | 541 | 590 | 23 | |
| Beetroot | 37 | DFAME | 514 | 619 | 460 | Forms dimer, can induce condensate |
| Squash | 62 | DFQL-1T | 516 | 654 | 56 | Tissue imaging capability |
Laboratory supplies
1. Glass-bottom, 24-well plate (VWR, catalog number: 82050-898) or 4-well dish (VWR, catalog number: 890135-976)
2. Microcentrifuge tubes, 1.5 mL (USA Scientific, catalog number: 1615-5500)
3. Sterile centrifuge tube, 15 mL (CELLTREAT, catalog number: 667015B)
4. Sterile centrifuge tube, 50 mL (CELLTREAT, catalog number: 229421)
5. Parafilm (Bemis, catalog number: PM999)
6. T-75 flasks w/ filter cap (USA Scientific, catalog number: CC7682-4875)
7. Serologic pipette, 2 mL individually wrapped (Fisher, catalog number: 13-678-11C)
8. Serologic pipette, 5 mL individually wrapped (Fisher, catalog number: 13-678-11D)
9. Serologic pipette, 10 mL individually wrapped (Fisher, catalog number: 13-678-11E)
10. Serologic pipette, 25 mL individually wrapped (Fisher, catalog number: 13-678-11)
11. Serologic pipette, 50 mL individually wrapped (Fisher, catalog number: 13-678-11F)
12. Pipette tips w/ filters, P2 (Denville, catalog number: 1159M41)
13. Pipette tips w/ filters, P20 (Denville, catalog number: 1159M43)
14. Pipette tips w/ filters, P200 (Denville, catalog number: 1159M40)
15. Pipette tips w/ filters, P1000 (Denville, catalog number: 1159M42)
Equipment
1. Certified type A2 biosafety cabinet (VWR, catalog number: 89413-128)
2. Pipette, single-channel P2 (Eppendorf, model: Research Plus)
3. Pipette, single-channel P20 (Eppendorf, model: Research Plus)
4. Pipette, single-channel P200 (Eppendorf, model: Research Plus)
5. Pipette, single-channel P1000 (Eppendorf, model: Research Plus)
6. Pipette fillers (ThermoFisher, model: S1 pipet fillers)
7. 4 °C refrigerator (Summit, model: FF7BIADA)
8. -40 °C ultra-low temperature freezer (VWR, catalog number: 76307-990)
9. -80 °C ultra-low temperature freezer (ThermoFisher, model: RLE50086A)
10. Water jacket tissue-culture incubator (VWR, model: 3015)
11. Biological microscope (Bioimager, model: BIM500FL)
12. Cell counter (Corning, catalog number: 6749)
13. Inverted wide-field fluorescence microscope (Nikon, model: Eclipse Ti-E)
14. Microcentrifuge (Eppendorf, model: 5424)
15. Centrifuge (Eppendorf, model: 5702R)
16. Cell counter (Corning, model: 6749)
17. Counting chamber (Corning, catalog number: 480200)
18. Water bath, 37 °C (Fisher Scientific, catalog number: 15-460-10)
19. Metallic water bath beads (Thomas Scientific, catalog number: 1220U61)
20. Stage top environmental chamber with flow control (Tokai Hit, model: INUTIZ)
21. Motorized stage (Nikon, model: TiSER)
22. High-numerical aperture (high-NA) fluorescence objective (Nikon, catalog model: CFI Plan Lambda Apo, 60×/1.4, oil)
23. sCMOS camera (Andor Technology, model: Zyla 5.5)
24. For imaging Hoechst 33342: DAPI filter cube with a sputter-coated excitation filter 395/25, dichroic mirror 425 (beamsplitter), and emission filter 460/50 (Chroma, model: 49028 ET)
25. For imaging Broccoli: FITC/GFP filter cube with a sputter-coated excitation filter 470/40, dichroic mirror 495 (beamsplitter), and emission filter 525/50 (Chroma, model: 49002 ET)
26. For imaging Corn and Squash: YFP filter cube with a sputter-coated excitation filter 500/20, dichroic mirror 515 (long pass), and emission filter 535/30 (Chroma, model: 49003 ET)
27. For imaging Squash: TRITC filter cube with a sputter-coated excitation filter 555/23, dichroic mirror 574 (beamsplitter), and emission filter 609/54 (Semrock, model: LED-TRITC-B-000)
28. For imaging mCherry: DsRed filter cube with a sputter-coated excitation filter 545/30, dichroic mirror 570 (long pass), and emission filter 620/30 (Chroma, model: 49005 ET)
29. For imaging SYTOXTM red: Cy5 filter cube with a sputter-coated excitation filter 620/60, dichroic mirror 660 (long pass), and emission filter 700/75 (Chroma, model: 49006 ET)
Software and datasets
1. ApE plasmid editor (https://jorgensen.biology.utah.edu/wayned/ape/) [23]
2. NUPACK nucleic acid analysis and design software (https://www.nupack.org/) [24]
3. UNAFold package (https://www.unafold.org/)
4. NIS Elements Advanced Research image acquisition software with High Content Analysis module (Nikon, version 5.4). Free alternative: ImageJ image analysis software with Fiji package (https://fiji.sc/), or similar image analysis software [25]
5. Prism graphing and statistical software (GraphPad, version 10). Free alternative: ggplot2 (https://ggplot2.tidyverse.org/) [26]
Procedure
文章信息
稿件历史记录
提交日期: Aug 20, 2025
接收日期: Sep 24, 2025
在线发布日期: Oct 24, 2025
出版日期: May 5, 2026
版权信息
© 2026 The Author(s); This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/).
如何引用
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- O’Hanlon, R. and Wu, K. Y. (2026). Visualizing diverse RNA functions in living cells with Spinach™ family of fluorogenic aptamers. Bio-protocol 16(9): e5504. DOI: 10.21769/BioProtoc.5504.
- Paige, J. S., Wu, K. Y. and Jaffrey, S. R. (2011). RNA mimics of green fluorescent protein. Science. 333(6042): 642–646. https://doi.org/10.1126/science.1207339
分类
细胞生物学 > 细胞成像 > 活细胞成像
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link












