- EN - English
- CN - 中文
Intracerebral Cannula Implantation in Mouse: A Proposed Method to Assess Glioblastoma Invasiveness and Serial Locoregional Treatment
小鼠脑内套管植入术:一种用于评估胶质母细胞瘤侵袭性及局部连续治疗的实验方法
发布: 2025年11月20日第15卷第22期 DOI: 10.21769/BioProtoc.5503 浏览次数: 1457
评审: Edgar Soria-GomezMario ValentinoAnonymous reviewer(s)
Abstract
Research on brain disorders, particularly in the field of oncology, requires in vivo models to evaluate various therapeutic approaches, including intracerebral drug delivery. To meet this requirement, the implantation of intracerebral cannulas offers a reliable method for administering candidate therapeutics directly into the brain. This protocol describes a surgical technique for cannula implantation in mice, enabling repeated administration of therapeutic compounds in the context of glioblastoma treatment. The method was designed with an emphasis on using accessible, easy-to-handle, and sterilized tools to optimize surgical outcomes. Particular attention was also given to animal welfare, notably through refined procedures for asepsis, anesthesia, and postoperative care.
Key features
• This protocol requires specific equipment and surgical mice expertise.
• This protocol is dedicated to brain disorders and cancer models, with a particular emphasis on locoregional delivery of drugs and derivatives.
Keywords: Cannula (套管)Graphical overview
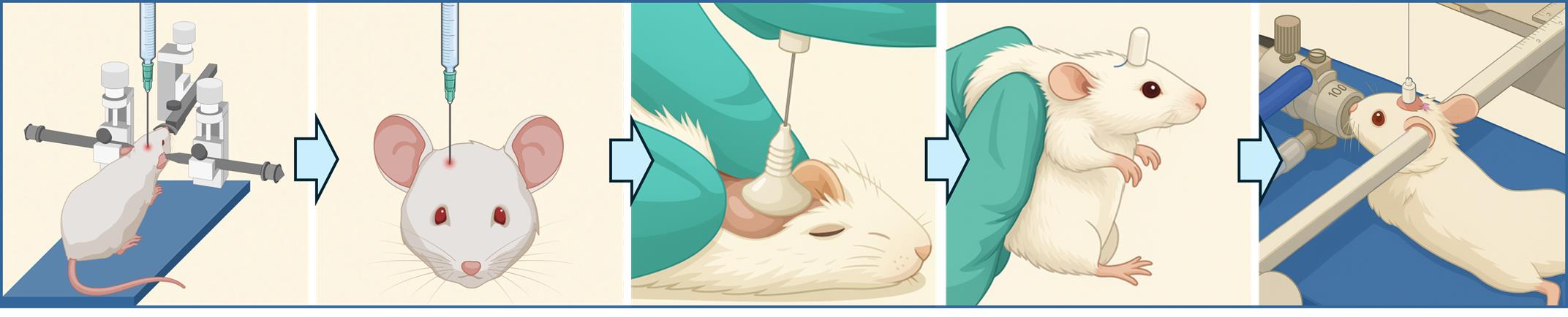
Intracerebral cannula implantation in mice for serial locoregional infusions
Background
Despite significant scientific advances in chemotherapy (when applicable), radiotherapy, and surgery, malignant tumors of the central nervous system continue to be associated with poor prognosis. In Belgium, the 5-year survival rate among adults remains as low as 22% [1,2]. New emerging treatments are increasingly offering targeted therapies. These therapies aim to modify the tumor microenvironment where the tumor settles and derives essential resources by altering the availability of oxygen and nutrients. More precise targeting of the tumor not only enhances therapeutic efficacy but also reduces off-target toxicity, which often significantly impacts patient quality of life.
The existence of preclinical models designed to evaluate the efficacy of these new therapies is a key element in providing therapeutic opportunities to patients through clinical trials. The implantation of an intracerebral cannula is the method of choice for repeated treatment at the level of the brain, and more specifically within the tumor [3]. Securing the cannula to the brain is a critical step for both the experimental approach and considerations in animal welfare. In this context, various fixation techniques are available, including the use of anchoring screws inserted into the top of the skull (calvaria) and secured with dental cement to anchor the base of the cannula. However, the use of screws is more suitable for rats, where the calvaria is sufficiently thick. In mice, the calvaria is thinner and less calcified, making screw insertion more difficult as there is a risk of damaging the integrity of the skull or the underlying brain tissue [4,5].
The choice of cement is therefore a critical factor in cannula implantation in mice, as the cement alone is responsible for securing the cannula in place. Typically, two types of dental cement are used: zinc polycarboxylate and methyl methacrylate (commonly referred to as pink cement). Zinc polycarboxylate is frequently associated with surgical complications, including skin necrosis, cannula detachment, and infection. Pink cement, although less reactive locally, contains a volatile hydrocarbon that can irritate the respiratory tract.
The use of dental cements that require reconstitution prior to application is relatively constraining as it involves multiple reagents, time-sensitive preparation, and prolonged drying times or UV-induced polymerization [5]. For ethical considerations, animal welfare, and efficient recovery, it is essential to select preparation methods that are as brief as possible to limit the duration of anesthesia. Finally, the polymerization of some cements can generate an exothermic reaction, releasing heat that may reach high temperatures. This thermal effect can lead to burns resulting in necrosis and gliosis (the proliferation of glial cells constituting the supporting tissue of the central nervous system), which remains a significant concern [4].
The protocol presented here describes a method for implanting an intracerebral cannula in mice. This device enables the direct infusion of therapeutic agents directly into the brain, or specifically into the site of a brain tumor that was previously initiated in the animal.
Materials and reagents
Biological materials
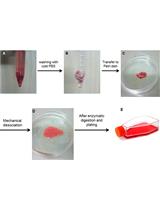
1. Balb/cj mice cadavers (Charles River, France)
2. Nod-scid gamma mice, females, 11–12 weeks old (Charles River, France)
3. U87-MG-Luc2 cells (ATCC, Manassas, Virginia, USA, ref: HTB14-LUC2TM)
Reagents
1. Buprenorphine (Vétergesic® 0.3 mg/mL) (Labiana Life Sciences, SA.N Barcelona, Spain)
2. Anti-inflammatory meloxicam (Metacam® 2 mg/mL) (Boehringer Ingelheim Animal Health France, Lyon, France)
3. Lidocaine hydrochloride 1% v/v (10 mg/mL) (Aspen France)
4. Ophthalmic gel (Vidisic®, 2 mg/g) (Dr Gerhard Mann Chem.-Pharm. Fabrik GmbH, Berlin, Germany)
5. Isoflurane (Isoflutek® 1,000 mg/g) (Laboratorios Karizoo, Barcelona, Spain)
6. Chlorhexidine digluconate soap (Clorexyderm® 4%) (MP Labo, Grasse, France)
7. Chlorhexidine digluconate solution diluted in water (Coditane® 50%) (Fraver sa, Kontich, Belgium)
8. Ethanol 70% (diluted in distilled water)
9. Phosphate buffered saline (PBS) (Gibco, catalog number: 392-034)
10. Minimum essential medium (MEM) (Gibco, catalog number: 31095-029)
11. Fetal bovine serum (FBS) (Gibco, catalog number: 10270106)
12. Penicillin-Streptomycin (10,000 U/mL) (Thermo Fisher, catalog number: 15140122)
13. Puromycin dihydrochloride (10 mg/mL) (Thermo Fisher, catalog number: A1113803)
14. TrypLETM Express Enzyme (1×), no phenol red (Thermo Fisher, catalog number: 12604013)
15. NaCl 0.9% (9 mg/mL) (Laboratories Sterop, Brussels, Belgium)
Equipment
1. Guide cannula-single/O.D.0. 48 mm-26G/pedestal 6 mm/3.5, autoclave up to 121 °C (C = 2.5MM, RWD®, catalog number: 62003)
2. Cap-single/O.D.0.30 mm/M3.5 (RWD®, catalog number: 62102, G2 = 0.5MM)
3. Dental cement G-Cem One kit, and provided Loctite (GC G-CEM ONETM, GC Europe NV)
4. Micro-CT scan (Brüker Skyscan, catalog number: 1276)
5. Mini-electric trimmer (Aesculap Schermaschinen® GmbH, catalog number: GT608, 004613)
6. Cotton swabs (Deltalab, Barcelona, Spain, catalog number: 300200)
7. Size 15 scalpel blades (Avantor, Radnor, PA, USA, catalog number: 233-0025)
8. Surgical scissors and forceps (Fine Science Tools, Heidelberg, Germany, catalog numbers: 14064-11, 91108-12)
9. Black marker
10. Microdrill (RWD®, catalog number: 78001)
11. Drill bits (RWD®, HM1008 0.8 mm, round tip, catalog number: 78042)
12. Thermostar temperature controller with heating pad included (RWD®, catalog number: 69026)
13. Standard digital stereotaxic frame with digital readout (RWD®, catalog number: 68803)
14. Anesthesia adaptor for 20–30 g mouse, tube with nosecone mask (RWD®, catalog number: 68077)
15. Standard probe holder, corner type, 0.3–1.5 mm (RWD®, catalog number: 68201)
16. Cannula holder (RWD®, catalog number: 68217)
17. Pair of 60° non-rupture ear bars for mouse (RWD®, catalog number: 68306)
18. Legato 130 single syringe (KD Scientific, Holliston, USA, order code: 788100)
19. Hamilton syringe 10 μL, 700 series with removable needle and 26s needle size, sharp tip (Merck, catalog number: 20697)
20. Hamilton needle, 32G needle size (Hamilton Company, catalog number: 32/51/3)
21. Suture thread 5/0 or 6/0 (VicrylTM)
22. Sterile surgical field (Lohmann & Rauscher, 45 × 75 cm sterile)
23. T75 flask (Sarstedt, catalog number: 833911002)
24. T175 flask (Sarstedt, catalog number: 833911)
Procedure
文章信息
稿件历史记录
提交日期: Aug 27, 2025
接收日期: Oct 8, 2025
在线发布日期: Oct 22, 2025
出版日期: Nov 20, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Henry, A., Stordeur, P., Lapierre, A., Buttenaers, C., Lefevre, M., Noelanders, M., Mottart, C., Delincé, L., Destiné, S., Etienne, Q., Nijskens, C., Filee, P. and Thirion, G. (2025). Intracerebral Cannula Implantation in Mouse: A Proposed Method to Assess Glioblastoma Invasiveness and Serial Locoregional Treatment. Bio-protocol 15(22): e5503. DOI: 10.21769/BioProtoc.5503.
分类
神经科学 > 神经系统疾病 > 脑肿瘤 > 多形性胶质母细胞瘤
癌症生物学 > 侵袭和转移 > 癌症治疗
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link