- EN - English
- CN - 中文
Spheroid Sheets: A Scalable Platform for Producing Tissue Membrane Constructs
球状细胞片:可规模化生产组织膜结构的平台
(§ Technical contact) 发布: 2025年11月20日第15卷第22期 DOI: 10.21769/BioProtoc.5501 浏览次数: 1533
评审: Elena A. OstrakhovitchAnonymous reviewer(s)
Abstract
Bottom-up tissue engineering using cell spheroids offers many advantages in recapitulating native cell–cell and cell–matrix interactions. Many tissues, such as cartilage, bone, cardiac muscle, intestine, and neural tissues, have been tissue-engineered using cell spheroids. However, previous methods for spheroid assembling, such as mold casting, hydrogel-based bioprinting, or needle array, either lack control over final tissue geometry or face challenges in scalability and throughput. In this protocol, we describe a robust and scalable tissue engineering method for assembling cell spheroids into a thin, planar spheroid sheet. The spheroids are sandwiched between two flexible meshes held by a frame, facilitating uniform spheroid fusion while ensuring nutrient exchange and ease of handling. We demonstrate this method by producing thin cartilage tissue from human mesenchymal stem cells undergoing chondrogenic differentiation. This approach offers a practical platform for producing thin membrane-like tissue constructs for many research and therapeutic applications.
Key features
• Spheroid-based tissue engineering: Utilizing cell spheroids to build various membrane-like tissues.
• Controlled tissue thickness: Frame and mesh constrain thickness and guide lateral fusion of spheroids, enabling uniform and thin tissue for efficient nutrient diffusion.
• Scaffold-free construct: After the thin tissue membrane is formed, the frame and mesh can be removed.
• Mechanical support: Meshes enable easy handling and can aid in transplantation of the constructs, for example, by allowing them to be wrapped or sutured.
Keywords: Cell spheroids (细胞球体)Graphical overview
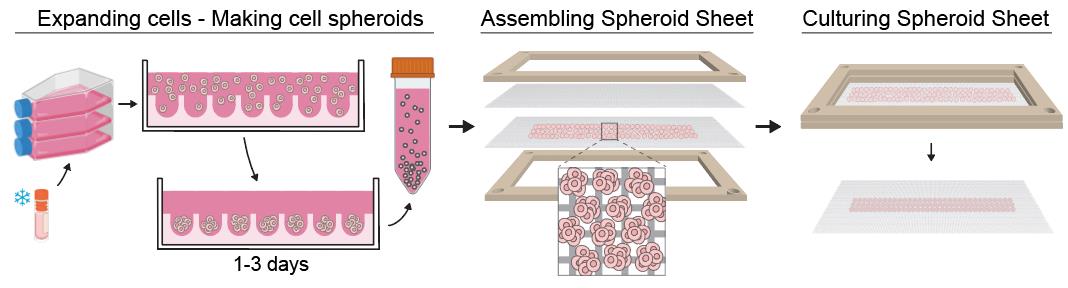
Workflow for producing a spheroid sheet. Cell spheroids of a specific size are made using microwell plates. Next, the spheroids are harvested and seeded in a thin homogenous layer on a mesh, followed by placement of a second mesh to sandwich the spheroids in between. The two meshes are secured using a frame, which maintains the assembly during in vitro culture. Spheroids fuse laterally into a thin tissue, allowing efficient nutrient and growth factor diffusion during culture.
Background
Tissue engineering (TE) is an interdisciplinary field that aims to grow functional tissue constructs in the laboratory for the repair, replacement, or enhancement of damaged tissue or organs in human patients. Within TE, two broad strategies are commonly employed. The top-down approach involves seeding cells, often single cells, onto a biomimetic scaffold, which is designed to mimic key structural and mechanical features of the target tissue. In contrast, the bottom-up approach assembles tissues from modular building blocks, such as cell spheroids, leveraging their self-assembly to form larger and more complex tissue constructs [1].
Three main methods for assembling cell spheroids have been described in the literature: (i) placing spheroids in contact within a mold (mold-based assembly), (ii) bioprinting with spheroids or positioning spheroids within a hydrogel matrix using bioprinting (hydrogel-assisted bioprinting), and (iii) physically securing spheroids on a fine needle array (Kenzan method) [2,3]. The mold-based approach is most common but often fails to achieve the intended size and shape of the tissue construct due to uncontrolled fusion of the spheroids into a larger mass [4]. Hydrogel-assisted bioprinting allows precise placement but can reduce direct spheroid-spheroid contact, leading to incomplete fusion. The Kenzan method is an elegant technique, but its throughput and scalability are limited [5].
Our spheroid assembly method involves using two meshes to constrain the cell spheroids within a thin layer, facilitating their fusion into a membrane-like tissue, which we term a spheroid sheet [6]. A rigid frame keeps the spheroid sheet flat and thin during culture. By maintaining a thin layer, we enhance the diffusion of medium and growth factors, thereby promoting the formation of a thin and uniform tissue membrane. The mesh should be made of bioinert material to promote spheroid self-assembly, be mechanically strong to be kept under tension during culture, and be highly porous for optimal diffusion.
This protocol describes detailed steps for making cartilage tissue membranes from human mesenchymal stem cell (hMSC) spheroids undergoing chondrogenic differentiation in vitro. Researchers can adapt this spheroid sheet protocol by using other cell types and differentiation media to produce a variety of tissue types with the thin membrane form factor.
Materials and reagents
Biological materials
Human mesenchymal stem cells (hMSC) can be purchased from cell banks such as Lonza (catalog number: PT-2501), PromoCell (catalog number: C-12977), or RoosterBio (catalog number: MSC-031). hMSCs can be derived from either bone marrow, adipose, or umbilical cord tissue. Researchers should obtain a certificate of analysis from the cell bank, showing successful trilineage differentiation for the purchased lot of hMSCs.
Reagents
1. Sodium phosphate dibasic (Sigma-Aldrich, Merck, catalog number: 255793)
2. Heparin sodium salt from porcine intestinal mucosa (Sigma-Aldrich, Merck, catalog number: H3149)
3. Bovine serum albumin (BSA) (Sigma-Aldrich, Merck, catalog number: A9418)
4. Human FGF-acidic (FGF-1) protein (Thermo Fisher Scientific, catalog number: 100-17A-500)
5. Citric acid (Sigma-Aldrich, Merck, catalog number: C2404)
6. 2-Phospho-L-ascorbic acid trisodium salt (ascorbic acid) (Sigma-Aldrich, Merck, catalog number: 49752)
7. L-Proline (Sigma-Aldrich, Merck, catalog number: P5607)
8. Dexamethasone (Sigma-Aldrich, Merck, catalog number: D4902)
9. Human TGF-beta 1 (TGF-β1) protein (Thermo Fisher Scientific, catalog number: 100-21-500)
10. DPBS, no calcium, no magnesium (Thermo Fisher Scientific, Gibco, catalog number: 14190144)
11. TrypLE Express enzyme (1×), no phenol red (Thermo Fisher Scientific, Gibco, catalog number: 12604013)
12. Fetal bovine serum (FBS) (Thermo Fisher Scientific, Gibco, catalog number: A3160502)
13. Penicillin-streptomycin (Thermo Fisher Scientific, Gibco, catalog number: 15140122)
14. DMEM, low glucose (Thermo Fisher Scientific, Gibco, catalog number: 10567014)
15. DMEM, high glucose (Thermo Fisher Scientific, Gibco, catalog number: 10569010)
16. Insulin-transferrin-selenium-ethanolamine (ITS-X) (Thermo Fisher Scientific, Gibco, catalog number: 51500056)
Solutions
1. Expansion medium for hMSCs (see Recipes)
a. FGF-heparin (1,000×) stock
2. Chondrogenic medium for hMSCs (see Recipes)
a. Ascorbic acid (500×) stock
b. Proline (1,000×) stock
c. Dexamethasone (1,000×) stock
d. TGF-β1 (1,000×) stock
Recipes
1. Expansion medium for hMSCs
| Reagent | Final concentration | Volume |
|---|---|---|
| DMEM, low glucose | n/a | 500 mL |
| Penicillin-streptomycin | Penicillin 90 U/mL; streptomycin 90 μg/mL | 5 mL |
| FBS | 9% (v/v) | 50 mL |
| FGF-heparin (1,000×) (see below) | [FGF-1] = 9 ng/mL; [heparin] = 4.5 μg/mL | 0.5 mL |
a. FGF-heparin (1,000×) stock
| Step | Reagent and amount | Concentration | Procedure note |
| 1. Prepare sodium phosphate buffer | 3.55 mg of sodium phosphate dibasic + 5 mL of distilled water | [Na2HPO4] = 5 mM | Adjust pH to 8.0, filter through a 0.22 μm filter |
| 2. Prepare heparin-BSA buffer | 250 mg of heparin + 50 mg of BSA + 50 mL of DPBS | [Heparin] = 5 mg/mL, [BSA] = 0.1% w/v | Filter through a 0.22 μm filter |
| 3. Dissolve FGF-1 with sodium phosphate buffer | 1 mL of sodium phosphate buffer + FGF-1 (500 μg) | [FGF-1] = 500 μg/mL in Na2HPO4 | Aseptically add 1 mL of Na2HPO4 to the FGF-1 vial (500 μg) and pipette to completely dissolve the powder |
| 4. Dilute FGF-1 with heparin-BSA buffer; aliquot | 1 mL of FGF-1 (500 μg/mL) + 49 mL of heparin-BSA buffer | [FGF-1] = 10 μg/mL, [heparin] = ~5 mg/mL | Mix thoroughly and rest for 15 min at room temperature. Aseptically aliquot (550 μL) and store at -80 °C. |
2. Chondrogenic medium for hMSCs
| Reagent | Final concentration | Volume |
| DMEM high glucose | n/a | 488 mL |
| Penicillin-streptomycin | Penicillin 100 U/mL; streptomycin 100 μg/mL | 5 mL |
| ITS-X (100×) | 1× | 5 mL |
| Ascorbic acid (500×) (see below) | 0.2 mM | 1 mL |
| Proline (1,000×) (see below) | 40 μg/mL | 0.5 mL |
| Dexamethasone (1,000×) (see below) | 100 nM | 0.5 mL |
| *TGF-β1 (1,000×) (see below) | 10 ng/mL | Dilute 1,000 times in medium |
*Except for TGF-β1, all other components of the chondrogenic medium can be combined and stored at 4 °C. Immediately before use, freshly thawed TGF-β1 is added to the required volume of medium to make the complete chondrogenic medium.
a. Ascorbic acid (500×) stock
| Step | Reagent and amount | Concentration | Procedure note |
|---|---|---|---|
| Dissolve ascorbic acid, filter, and aliquot | 644 mg of ascorbic acid + 20 mL of distilled water | [Ascorbic acid] = 32.2 mg/mL (100 mM) | Filter through a 0.22 μm filter, aliquot (1 mL), and store at -80 °C |
b. Proline (1,000×) stock
| Step | Reagent and amount | Concentration | Procedure note |
|---|---|---|---|
| Dissolve proline, filter, and aliquot | 400 mg of L-proline + 20 mL of distilled water | [L-Proline] = 20 mg/mL | Filter through a 0.22 μm filter, aliquot (0.5 mL), and store at -80 °C |
c. Dexamethasone (1,000×) stock
| Step | Reagent and amount | Concentration | Procedure note |
|---|---|---|---|
| 1. Dissolve dexamethasone | 25 mg of dexamethasone + 25 mL of 100% ethanol | [Dexamethasone] = 1 mg/mL or 2.55 mM | Store this solution at -80 °C for subsequent uses |
| 2. Dilute dexamethasone, aliquot | 392 μL of dexamethasone (2.55 mM) + 9.6 mL of 100% ethanol | [Dexamethasone] = 0.1 mM | Filter through a 0.22 μm filter, aliquot (0.5 mL), and store at -80 °C |
d. TGF-β1 (1,000×) stock
| Step | Reagent and amount | Concentration | Procedure note |
|---|---|---|---|
| 1. Prepare citric acid buffer | 9.6 mg of citric acid + 5 mL of distilled water | [Citric acid] = 10 mM | Adjust pH to 3.0, filter through a 0.22 μm filter |
| 2. Prepare BSA buffer | 50 mg of BSA + 50 mL of DPBS | [BSA] = 0.1% w/v | Filter through a 0.22 μm filter |
| 3. Dissolve TGB-β1 with citric acid buffer | 1 mL of citric acid buffer + TGF-β1 (500 μg) | [TGF-β1] = 500 μg/mL in citric acid buffer | Aseptically add 1 mL of citric acid buffer to the TGF-β1 vial and pipette to completely dissolve the powder |
| 4. Dilute TGF-β1 with BSA buffer, aliquot | 1 mL of TGF-β1 (500 μg/mL) + 49 mL of BSA buffer | [TGF-β1] = 10 μg/mL | Mix thoroughly and rest for 15 min at room temperature. Aseptically aliquot (100 μL) and store at -80 °C. |
Laboratory supplies
1. Cell culture flasks with surface treated for adherence cells
2. 6-well plates or 10-cm Petri dishes
3. Microwell plates for making cell spheroids (e.g., Corning, catalog number: 4440 or Stemcell Technologies, catalog number: 34821). Alternatively, researchers can make agarose microwell plates using molds, such as MicroTissues 3D (Sigma-Aldrich, Merck, catalog number: Z764000)
4. Meshes can be obtained commercially or fabricated in-house. In this protocol, we use nylon meshes punched from biopsy bags (Simport Scientific, catalog number: M478), with inner pore dimensions of 150 μm × 200 μm. Circular meshes were cut using a Dumbbell Cutter (model SDL-100, ø 22-mm cutting die, Dumbbell Co., Japan)
5. Stainless-steel forceps, micro spatula with flat end, scalpel blade, ruler
6. Whatman filter paper (Sigma-Aldrich, Merck, catalog number: WHA1001090)
7. Frame for spheroid sheet assembly; this can be 3D-printed and consists of two parts (bottom and top) that, when clamped together, apply tension to the meshes to keep them flat (Figure 1). Design recommendations:
a. Dimensions: For spheroid sheets up to 2 cm × 2 cm, the frame can be designed to fit a standard 6-well plate by keeping the outer diameter ≤3 cm. A minimum thickness of 2 mm is recommended for adequate stiffness. The spheroid sheet window can be customized to the desired size and shape (e.g., circular or rectangular) and may include a chamfered edge to facilitate the removal of trapped bubbles beneath the mesh. For larger spheroid sheets, the design can be scaled up to fit a standard 10-cm Petri dish or larger.
b. Locking mechanism: The two parts may be secured using either a friction fit or a latch mechanism. For the latch design, extend portions of the bottom part’s wall inward by 0.5–1 mm with a 50° overhang to enable printing without support. The latch thickness should be at least 2 mm for adequate strength, and the top edge should be chamfered to allow the top part to slide over easily. For larger constructs, screws can be used to provide uniform pressure on the mesh.
c. Handling features: Incorporate small holes or protrusions in the frame to facilitate aseptic handling with forceps/tweezers (e.g., opening/closing the two parts, lifting the frame within a 6-well plate)
d. Low-profile design: Keep the frame low profile so minimal medium is required to submerge it; position the spheroid-sheet window lower to enable easy microscopic observation.
e. Standoffs (legs): Add short protrusions on the underside to slightly elevate the frame, preventing a tight seal with the well bottom and ensuring medium access beneath the mesh. In our design (Figure 1), the latches double as the legs, since the frame is flipped before being placed into the 6-well plate.
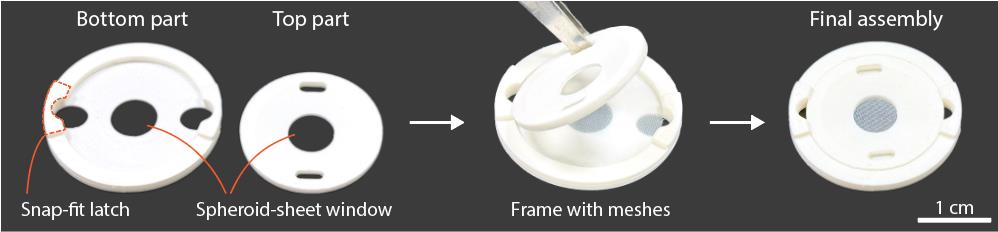
Figure 1. Example of a frame for spheroid-sheet assembly. The frame is designed to secure the meshes and spheroid construct, maintaining them in a flat configuration to keep the spheroid layer thin for efficient diffusion.
Equipment
1. Cell incubator (5% CO2, 100% humidity)
2. Biosafety cabinet (BSC) for aseptic handling
3. Benchtop centrifuge with adaptor for well plates
4. 3D printer, such as a fused deposition modeling (FDM) printer
Procedure
文章信息
稿件历史记录
提交日期: Aug 26, 2025
接收日期: Oct 8, 2025
在线发布日期: Oct 17, 2025
出版日期: Nov 20, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Le, Q. B., Ezhilarasu, H., Chan, W. W. and Choudhury, D. (2025). Spheroid Sheets: A Scalable Platform for Producing Tissue Membrane Constructs. Bio-protocol 15(22): e5501. DOI: 10.21769/BioProtoc.5501.
分类
生物工程 > 生物医学工程
细胞生物学 > 细胞工程 > 组织工程
干细胞 > 成体干细胞 > 间充质干细胞
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link