- EN - English
- CN - 中文
In Vivo Retroviral Transduction of Cardiac Myofibroblasts Using Intramyocardial Injection Immediately Post-myocardial Infarction
心肌梗死后即刻进行心肌内注射,实现心脏肌成纤维细胞的体内逆转录病毒转导
发布: 2025年11月20日第15卷第22期 DOI: 10.21769/BioProtoc.5500 浏览次数: 1554
评审: Pilar Villacampa AlcubierreAnonymous reviewer(s)
Abstract
Following myocardial infarction (MI), myocardial cells undergo cell death, and the necrotic region is replaced by extracellular matrix (ECM) proteins such as collagens. Myofibroblasts are responsible for producing these ECM proteins. Cardiac myofibroblasts are differentiated from resident fibroblasts in response to inflammation. To date, genetically modified mice driven by the Periostin promoter and adeno-associated virus 9 (AAV9) carrying the Periostin promoter have been used for gene transfer into cardiac myofibroblasts. However, these methods require multiple steps and are time-consuming and expensive. Therefore, we developed a method for delivering genes into cardiac myofibroblasts using retroviruses. Specifically, the DNA of the target gene was transfected into Plat-E cells, which are packaging cells, to generate retroviruses. The virus-containing supernatant was then harvested, and the viruses were pelleted by centrifugation and suspended in PBS-containing polybrene. Subsequently, permanent occlusion of the left coronary artery was performed, and 20 μL of viral solution was immediately administered using a 29G needle at a position 1–2 mm below the ligation site in the heart of mice maintained in an open chest state. Using this method, we were able to introduce genes into the myofibroblasts of interest surrounding the MI site.
Key features
• Retroviruses are taken up only by proliferating cells, enabling highly specific gene transfer into myofibroblasts.
• Any gene incorporated into the genome by retroviruses will continue to be expressed over the long term, providing chronic in vivo evaluation.
• Myocardial injection targeting the infarct area of the left ventricle shows high infection efficiency in myofibroblasts.
• This protocol employs a very small-scale and simple virus concentration method.
Keywords: Intramyocardial injection (心肌内注射)Graphical overview
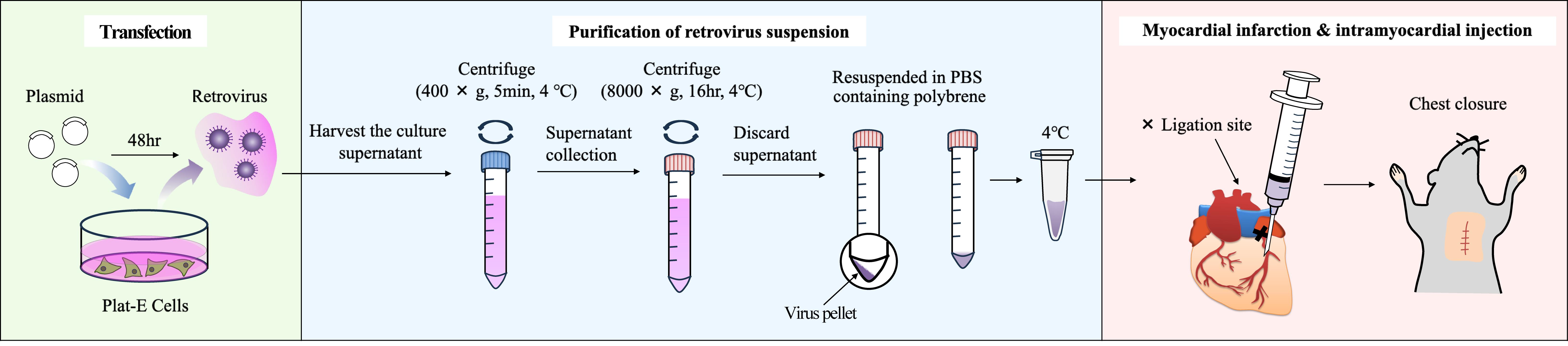
Background
To date, gene delivery technology to cardiac myofibroblasts has been performed by administering AAV9 or AAV-DJ constructs containing Cre recombinase driven by the promoter of Periostin, a marker gene for myofibroblasts, via the tail vein [1,2]. In addition, transgenic mice using the promoter of Periostin have been created as a method of introducing genes into cardiac myofibroblasts [3,4].
Keeping genetically modified mice is time-consuming and expensive. This protocol does not employ a promoter-driven strategy and does not require mouse breeding. The purification of AAV and other viruses requires multiple steps and an ultracentrifuge, which is expensive. Our protocol utilizes a relatively inexpensive floor-standing centrifuge and completes the concentration of the virus solution in a single centrifugation step. Therefore, this procedure can be readily performed by most researchers.
Considering that myofibroblasts in the fibrotic area have extremely high proliferative capacity and that retroviruses are readily incorporated into dividing cells, we considered that administering retroviruses to the heart after myocardial infarction (MI) treatment might be effective to introduce genes into myofibroblasts. Other cell types, such as endothelial cells, leukocytes, and pericytes, are also present in the fibrotic region, and it was assumed that these cells might also proliferate; fortunately, no genes were introduced into these cells. This is expected to be due to the lack of expression of the receptors required for retroviral infection in these cells.
We found that the protein Vestigial-Like Family Member 3 (VGLL3) is specifically expressed in cardiac myofibroblasts after MI [5]. Interestingly, experiments using isolated cardiac myofibroblasts revealed that VGLL3 translocated from the cytoplasm to the nucleus depending on mechanical stimulus. To verify this in vivo using intramyocardial injection immediately post-MI, EGFP-VGLL3 was expressed only in myofibroblasts in the fibrotic region, where the tissue stiffness is increased by the accumulation of collagens and localized to the nucleus. These results indicate that VGLL3 is a mechanosensitive protein in myofibroblasts in fibrotic hearts.
This protocol is expected to be applied in various fields of research on heart diseases involving cardiac myofibroblasts. Below, we introduce the retroviral transduction protocol of cardiac myofibroblasts using intramyocardial injection immediately post-MI.
Materials and reagents
Biological materials
1. Plat-E cells; cells containing gag, pol, and env genes, allowing retroviral packaging with a single plasmid transfection were provided by Dr. Kitamura, University of Tokyo, Japan. Plat-E cells are also commercially available (Cell Biolabs, catalog number: RV-101)
2. Wild-type house mouse (Mus musculus) (Japan SLC, catalog number: C57BL/6JmsSlc)
Reagents
1. Serum-free DMEM (Nacalai Tesque, catalog number: 08458-16)
2. FBS (Gibco, catalog number: 10270-106)
3. Penicillin-streptomycin (Nacalai Tesque, catalog number: 09367-34)
4. PEI-Max (Polysciences, catalog number: 24765-1)
5. Water deionized and sterilized (Nacalai Tesque, catalog number: 06442-95)
6. NaOH (Nacalai Tesque, catalog number: 31511-05)
7. Polybrene (Sigma-Aldrich, catalog number: 107689-10G)
8. Phosphate buffered saline (PBS) (Nacalai Tesque, catalog number: 14249-95)
9. Evans Blue (WAKO, catalog number: 056-04061)
10. EGFP-VGLL3/pMXs-puro [constructed in our laboratory; pMXs-puro retroviral vector (Cell Biolabs, catalog number: RTV-012)]
Solutions
1. Culture medium (see Recipes)
2. PEI Max solution (see Recipes)
3. Plasmid solution (see Recipes)
Recipes
1. Culture medium
| Reagent | Final concentration | Volume (mL) |
|---|---|---|
| Serum-free DMEM | 89% (v/v) | 445 |
| FBS (inactivation) | 10% (v/v) | 50 |
| Penicillin-streptomycin | 1% (v/v) | 5 |
| Total | n/a | 500 |
Prior to addition, inactivate FBS by heating at 56 °C for 30 min in a water bath. Then, filter it with a 0.2 μm filter.
Store the culture medium at 4 °C. Stable for at least one month.
2. PEI Max solution
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Water deionized and sterilized | n/a | 40 mL |
| PEI-Max | 1 μg/μL | 0.04 g |
| Total | n/a | 40 mL |
After mixing the reagents, adjust the pH to 7.4 with NaOH. Store the solution at 4 °C. Stable for at least 6 months.
3. Plasmid solution
| Reagent | Final concentration | Volume (μL) |
|---|---|---|
| Serum-free DMEM | 94% (v/v) | 564 |
| Plasmid (EGFP-VGLL3/pMXs-puro) | 10 μg/mL | 6 |
| PEI Max solution (Recipe 2) | 50 ng/mL | 30 |
| Total | n/a | 600 |
Prepare just before use. Use high-quality plasmid DNA (e.g., purified using a cesium chloride gradient or an endotoxin-free maxiprep kit) at a concentration of 1 μg/μL.
Laboratory supplies
1. Pipette tips, 1,000 μL (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 2179-HR)
2. Pipette tips, 200 μL (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 2069-HR)
3. Pipette tips, 20 μL (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 2149P-HR)
4. Pipette tips, 10 μL (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 2140-HR)
5. Filter units (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 569-0020)
6. 10-cm cell culture dish (Nippon Genetics, catalog number: FG-2090)
7. 15 mL tube (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 339650)
8. 15 mL tube (AS ONE Corporation, catalog number: 1-3500-01)
9. Surgical tape (3M, catalog number: 1527-0)
10. Razor blade (FEATHER, catalog number: FH-10)
11. 8-0 braided silk (NATSUME SEISAKUSHO, catalog number: M6-80B2)
12. 5-0 braided silk (NATSUME SEISAKUSHO, catalog number: ER12-50B1)
13. 29G 0.5 mL syringe (TERUMO, catalog number: SS-10M2913)
14. 1.7 mL tube (BM Bio, catalog number: BM-15)
Equipment
1. Pipetman 1,000 μL (Gilson, catalog number: F123602)
2. Pipetman 200 μL (Gilson, catalog number: F123601)
3. Pipetman 20 μL (Gilson, catalog number: F123600)
4. Aspirator (TOKYO RIKAKIKAI, catalog number: A1000S)
5. Respirator (Shinano Manufacturing, catalog number: SN-480-7X2T)
6. Heating pad (Marukan, catalog number: RH-207)
7. Optical microscope (Olympus, catalog number: SZX7)
8. Tweezer (NATSUME SEISAKUSHO, catalog number: A-12-2)
9. Tweezer (MEISTER, catalog number: 2-AXAL)
10. Small scissors (NATSUME SEISAKUSHO, catalog number: B-12)
11. Forceps (NATSUME SEISAKUSHO, catalog number: C-1)
12. Clean bench (Panasonic Healthcare, catalog number: MCV-B131S)
13. Water bath (TAITEC, catalog number: Personal-11)
14. Centrifuge (TOMY SEIKO, catalog number: LC-200)
15. Centrifuge (TOMY SEIKO, catalog number: AX-321)
16. CO2 incubator (SANYO, catalog number: MCO-18AIC)
Procedure
文章信息
稿件历史记录
提交日期: Aug 19, 2025
接收日期: Oct 8, 2025
在线发布日期: Oct 21, 2025
出版日期: Nov 20, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/).
如何引用
Ono, S., Watanabe, H., Horii, Y. and Nakaya, M. (2025). In Vivo Retroviral Transduction of Cardiac Myofibroblasts Using Intramyocardial Injection Immediately Post-myocardial Infarction. Bio-protocol 15(22): e5500. DOI: 10.21769/BioProtoc.5500.
分类
医学 > 心血管疾病
细胞生物学 > 基于细胞的分析方法 > 基因表达
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link