- EN - English
- CN - 中文
Labeling Postsynaptic Densities for Super-Resolution Microscopy With Minimal Signal-Loss and Offset
低信号损失与低偏移的突触后致密区超分辨显微标记方法
(*Contributed equally to this work; §Technical contact: shengyang.ho@berkeley.edu) 发布: 2025年11月05日第15卷第21期 DOI: 10.21769/BioProtoc.5499 浏览次数: 1570
评审: Olga KopachAnonymous reviewer(s)
Abstract
Accurate labeling of excitatory postsynaptic sites remains a major challenge for high-resolution imaging due to the dense and sterically restricted environment of the postsynaptic density (PSD). Here, we present a protocol utilizing Sylites, 3 kDa synthetic peptide probes that bind with nanomolar affinity to key postsynaptic markers, PSD-95 and Gephyrin. eSylites (excitatory Sylites) specifically target the PDZ1 and PDZ2 domains of PSD-95, enabling precise and efficient labeling of excitatory postsynaptic density (ePSD). In contrast, iSylites (inhibitory Sylites) bind to the dimerizing E-domain of the Gephyrin C-terminus, allowing selective visualization of inhibitory postsynaptic density (iPSD). Their small size reduces linkage error and enhances accessibility compared to conventional antibodies, enabling clear separation of PSD-95 nanodomains in super-resolution microscopy. The protocol is compatible with co-labeling using standard antibodies and integrates seamlessly into multichannel immunocytochemistry workflows for primary neurons and brain tissue. This method enables robust, reproducible labeling of excitatory synapses with enhanced spatial resolution and can be readily adapted for expansion microscopy or live-cell applications.
Key features
• Protocol for using synthetic bidendate peptide probes: eSylites [1] for PSD-95 at excitatory synapses and iSylites [2] for Gephyrin at inhibitory synapses.
• Compatible with standard antibody staining for multiplexed imaging of primary neurons and tissue sections.
• Reduces linkage error, improving spatial resolution and nanodomain visualization in super-resolution microscopy.
Keywords: Synaptic markers (突触标记物)Graphical overview
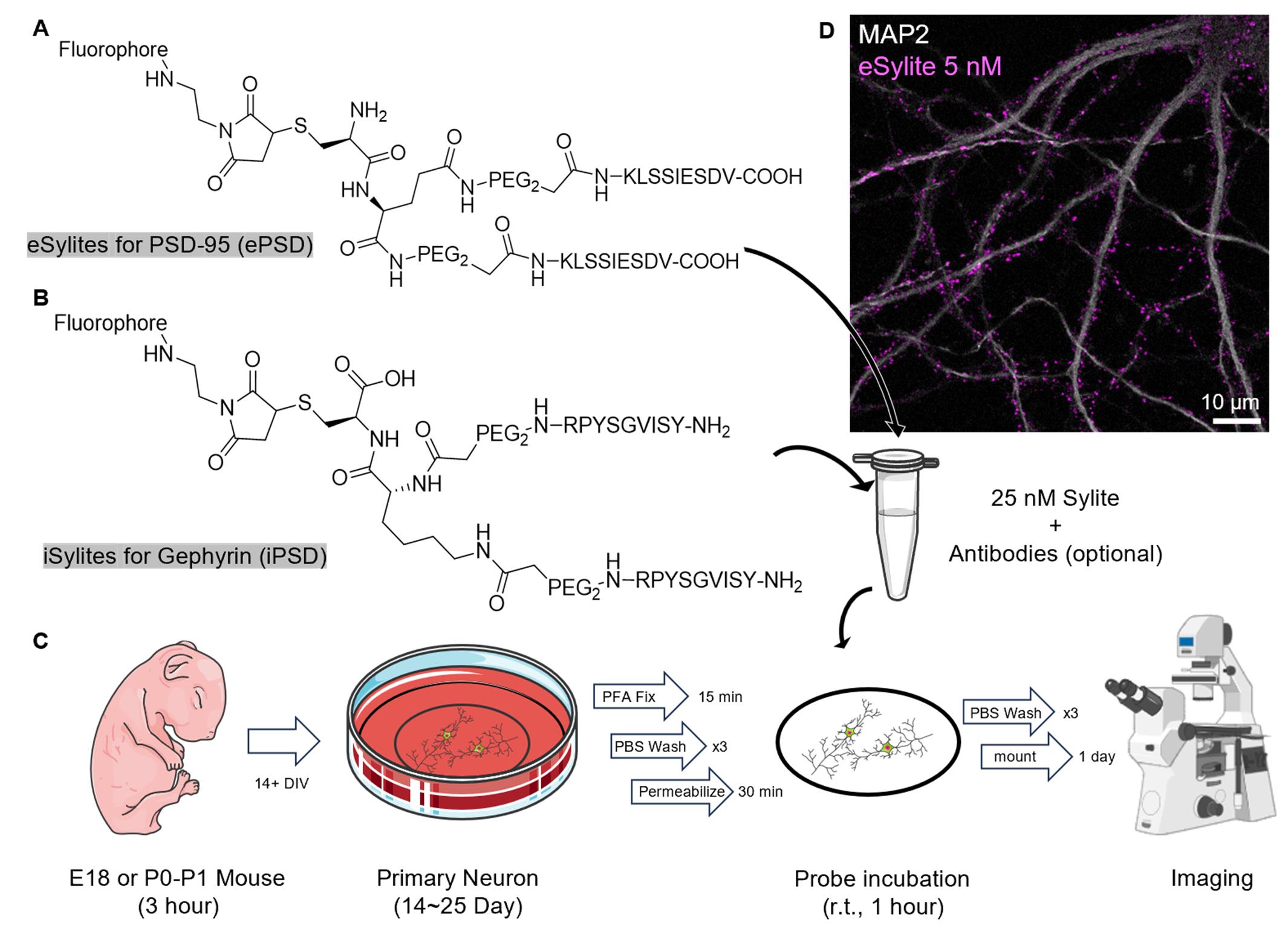
Graphical overview of the staining procedure. (A) Structure of eSylite targeting PSD-95 at the excitatory postsynaptic density (ePSD) [1]. (B) Structure of iSylite targeting Gephyrin at the inhibitory postsynaptic density (iPSD) (C) Schematic of Sylites application in fixed primary neuron imaging; more images can be found in Figure 2. Image adapted from Servier Medical Art (https://smart.servier.com/), licensed under CC BY 4.0 (https://creativecommons.org/licenses/by/4.0/). (D) Confocal image of 5 nM sulfoCy5-eSylite on 22 days in vitro (DIV) dissociated hippocampal neuron co-stained with MAP2.
Background
PSD-95 is a key scaffolding protein and a widely used postsynaptic marker for excitatory synapses. Within the postsynaptic density (PSD), PSD-95 assembles into dense nanodomains that recruit both AMPA and NMDA receptors in alignment with the presynaptic active zone, thereby facilitating and regulating efficient synaptic transmission. Dysregulation of PSD-95 has been associated with cognitive and learning deficits linked to neurodevelopmental disorders such as schizophrenia and autism [3].
The highly crowded environment of the PSD presents a major challenge for reliable antibody-based labeling, as the large size of full-length antibodies introduces significant steric hindrance and limits access to epitopes deep within the nanodomain. This often results in biased labeling toward peripheral molecules. Additionally, full-length antibodies introduce a linkage error of up to ~12 nm, and commonly used primary–secondary antibody complexes can extend this to ~24 nm. While negligible in diffraction-limited microscopy, such displacement becomes substantial in super-resolution techniques with sub-50 nm resolution.
To overcome these limitations, we developed Sylites using a peptide microarray approach [4]. Sylites are synthetic bidentate peptide probes with nanomolar affinity for key postsynaptic density markers: PSD-95 at excitatory synapses and Gephyrin at inhibitory synapses. Unlike conventional full-length antibodies (~150 kDa) or nanobodies (~15 kDa) [5], the ~3 kDa Sylite peptides minimize dye offset, significantly reducing linkage error and enabling precise, spot-on labeling for high-resolution microscopy. Their small size also greatly enhances tissue penetration and labeling efficiency, particularly within densely packed synaptic environments.
eSylites were designed by mimicking and dimerizing the C-terminal tail of GluN2B, a subunit of NMDA receptors. This confers nanomolar affinity and high specificity for the PDZ1 and PDZ2 domains of PSD-95 through avidity. We demonstrate that eSylites offer superior localization accuracy and structural resolution of postsynaptic nanodomains compared to conventional antibodies.
In parallel, iSylites were developed as dimers derived from the GlyR β subunit binding sequence, targeting the C-terminal E-domain of Gephyrin, which forms a dimeric structure. The modifications, together with avidity-based design, enhance binding affinity to Gephyrin up to 46,000-fold [6], enabling reliable and specific labeling of inhibitory postsynaptic densities. Together with compact synthetic probes developed by the Hamachi lab for GABAARs and GluRs [7], Sylites offer a complete toolbox for the offset-free labeling of inhibitory and excitatory neurotransmitter receptors, together with their underlying post-synaptic scaffolds.
Both eSylites and iSylites take advantage of the fractional occupancy of scaffolding protein binding sites [8,9]. While this functional selectivity is negligible in diffraction-limited imaging, it can reveal additional insights into the reserve pool of scaffold proteins when super-resolution techniques with resolution beyond 10 nm are applied. Currently, Sylites face limitations in direct stochastic optical reconstruction microscopy (dSTORM) imaging due to dense labeling, which may induce energy transfer mechanisms that compromise localization precision and photostability in dSTORM buffers [10]. Nevertheless, their fully synthetic origin and modular design make Sylites highly adaptable for specific experimental applications. In the future, advanced imaging techniques such as expansion microscopy (ExM) combined with DNA-PAINT or optimized dSTORM conditions may further enhance the utility of Sylites in nanoscale synaptic mapping.
Materials and reagents
Biological materials
1. P0–P1 mouse, C57BL/6J strain
Reagents
1. Sylites (iSylite: Nanotag, catalog number: P4001; eSylites and a range of conjugates are also available via mail to hans.maric@uni-wuerzburg.de and jwhell@ucdavis.edu
2. Poly-D-lysine (PDL) hydrobromide (Sigma-Aldrich, catalog number: P6407-5MG, CAS number: 27964-99-4)
3. UltraPureTM DNase/RNase-free distilled water (Thermo Fisher, catalog number: 10977015)
4. GibcoTM HBSS, no calcium, no magnesium (Thermo Fisher, catalog number: 14170112)
5. GibcoTM HBSS, calcium, magnesium (Thermo Fisher, catalog number: 24020117)
6. B-27TM Plus Supplement (50×) (Thermo Fisher, catalog number: A3582801)
7. NeurobasalTM Plus medium (Thermo Fisher, catalog number: A3582901)
8. GlutaMAXTM supplement (Thermo Fisher, catalog number: 35050061)
9. Trypsin-EDTA (0.05%), phenol red (Thermo Fisher, catalog number: 25300054)
10. Gentamicin solution (Sigma-Aldrich, catalog number: G1397-10ML)
11. PBS, pH 7.4 (Thermo Fisher, catalog number: 10010023)
12. PierceTM 16% formaldehyde (w/v), methanol-free (Thermo Fisher, catalog number: 28906)
13. Glyoxal 40 wt. % solution in water (Sigma-Aldrich, catalog number: 128465)
14. Fish serum blocking buffer (Thermo Fisher, catalog number: 37527)
15. Triton X-100 (Sigma-Aldrich, catalog number: T9284-100ML, CAS number: 9036-19-5)
16. ProLongTM Gold antifade mountant (Thermo Fisher, catalog number: P10144)
17. Paraformaldehyde, 16% w/v aqueous solution, methanol-free (Thermo Fisher, catalog number: 043368.9M)
18. Ethyl alcohol, pure (Sigma-Aldrich, catalog number: 459836-100ML)
19. Acetic acid, glacial (Sigma-Aldrich, catalog number: 695092-100ML)
20. NaOH (Fisher Scientific, catalog number: S318)
Solutions
1. PDL solution (see Recipes)
2. Culturing medium (see Recipes)
3. 4% paraformaldehyde (PFA) solution (see Recipes)
4. Glyoxal buffer (see Recipes)
5. Blocking and permeabilization buffer (see Recipes)
Recipes
1. PDL solution
5 mg of Poly-D-lysine hydrobromide in 50 mL of ultrapure water.
2. Culturing medium
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| B-27 plus supplement 50× | 1× | 1 mL |
| GlutaMAX 100× | 1× | 500 μL |
| Gentamicin | 5 μg/mL | 5 μL |
| Neurobasal Plus | n/a | 48.5 mL |
| Total | n/a | 50 mL |
Prepare and equilibrate in a CO2 incubator one day prior to dissection.
3. 4% PFA solution
1 mL of 16% PFA in 3 mL of PBS, pH 7.4.
4. Glyoxal buffer
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Ethyl alcohol, pure | 21.6% | 39.45 mL |
| Acetic acid glacial | 0.82% | 1.5 mL |
| Ultrapure water | n/a | 141.75 mL |
| 1 M NaOH | n/a | Titrate to pH 5 |
| Total | n/a | ~200 mL |
Add 0.856 mL of 40 wt. % glyoxal solution to 10 mL of glyoxal buffer prior to fixation [11].
5. Blocking and permeabilization buffer
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| PBS (1×) | 50% | 5 mL |
| Fish serum | 50% | 5 mL |
| Triton X-100 | 0.5% | 50 μL |
| Total | n/a | 10 mL |
For surface labeling with antibody, block without adding Triton X-100 and permeabilize after surface labeling.
Blocking is primarily for antibody co-staining. When using Sylites alone, serum (either fish serum or 3% BSA) can be omitted without introducing nonspecific labeling.
Laboratory supplies
1. Round coverslips, #1.5 18 mm (Warner Instrument, catalog number: 64-0714)
2. Microscope slides (Globe Scientific, catalog number: 1338)
3. 12-well plate (Greiner, catalog number: 665180)
4. T25 flask/50 mL conical tube (Corning, catalog number: 430639)
5. Straight forceps
6. 4” micro straight scissors, Castroviejo stainless steel (Electron Microscopy Sciences, catalog number: 72933)
8. Freer elevator/spatula
9. 60 mm Petri dish (Celltreat, catalog number: 229663)
10. Scalpel
11. Ultra-fine forceps (Excelta Corp, model: 7-SA)
12. Pipette tips
13. Parafilm
14. 150 mm Petri dish (Celltreat Scientific, catalog number: 229656)
Equipment
1. CO2 incubator (Heraeus HERAcell 240 CO2 Incubator)
2. Dissection microscope (AmScope, model: SM-1)
3. Spectrophotometer/NanoDrop (BioTek Synergy 2 with Take3 plate)
3. Fluorescence microscope (Leica, model: TCS SP8 gated STED 3× microscope)
Procedure
文章信息
稿件历史记录
提交日期: Jul 31, 2025
接收日期: Sep 28, 2025
在线发布日期: Oct 17, 2025
出版日期: Nov 5, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/).
如何引用
Ho, S., Huhn, C., Skeens, A., Hruska, M., Maric, H. M. and Hell, J. W. (2025). Labeling Postsynaptic Densities for Super-Resolution Microscopy With Minimal Signal-Loss and Offset. Bio-protocol 15(21): e5499. DOI: 10.21769/BioProtoc.5499.
分类
神经科学 > 细胞机理 > 突触生理学
细胞生物学 > 细胞信号传导 > 突触传递
细胞生物学 > 细胞染色
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link