- EN - English
- CN - 中文
Optimized Protocol for the Collection, Cryopreservation, and In Vitro Cultivation of Human Gut Microbiota for Toxicomicrobiomics Applications
用于毒理微生物组学研究的人体肠道微生物采集、冷冻保存与体外培养优化方法
发布: 2025年11月05日第15卷第21期 DOI: 10.21769/BioProtoc.5498 浏览次数: 1933
评审: Alba BlesaAnonymous reviewer(s)
Abstract
Xenobiotics, including environmental pollutants such as bisphenols, phthalates, and parabens, are widely present in food, cosmetics, packaging, and water. These compounds can reach the gastrointestinal tract and interact with the gut microbiota (GM), a complex microbial community that plays a key role in host immunity, metabolism, and barrier function. The GM engages in bidirectional communication with the host via the production of bioactive metabolites, including short-chain fatty acids, neurotransmitter precursors, and bile acid derivatives. Dysbiosis induced by xenobiotics can disrupt microbial metabolite production, impair gut barrier integrity, and contribute to the development of systemic disorders affecting distant organs such as the liver or brain. On the other hand, the GM can biotransform xenobiotics into metabolites with altered bioactivity or toxicity. In vitro models of the human GM offer a valuable tool to complement population-based and in vivo studies, enabling controlled investigation of causative effects and underlying mechanisms. Here, we present an optimized protocol for the collection, cryopreservation, and cultivation of human GM under strictly anaerobic conditions for toxicomicrobiomics applications. The method allows the assessment of xenobiotic–GM interactions in a cost-effective and ethically sustainable way. It is compatible with a wide range of downstream applications, including 16S rDNA sequencing, metabolomics, and endocrine activity assays. The protocol has been optimized to minimize oxygen exposure to less than 2 min, ensuring the viability of obligate anaerobes that dominate the gut ecosystem. This approach facilitates reproducible, mechanistic studies on the impact of environmental xenobiotics on human GM.
Key features
• Strict anaerobic handling of human fecal samples: The protocol maintains anaerobic conditions from collection to cultivation, with oxygen exposure limited to less than 2 min.
• Pooled-sample inoculum for reproducibility: Cryopreserved inoculum derived from pooled donor samples reduces inter-individual variability and ensures high reproducibility across experiments.
• Compatibility with diverse downstream applications: The protocol supports a wide range of analyses, including 16S rDNA sequencing, untargeted metabolomics, SCFA profiling, and host–GM interaction studies.
• High-throughput capacity: Up to 192 samples can be cultured simultaneously, enabling efficient large-scale experiments.
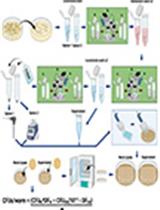
Keywords: Gut microbiota (肠道微生物群)Graphical overview
Background
In vitro models of gut microbiota (GM) cultures provide a valuable tool alongside in vivo studies and population-based research, as they enable the investigation of causal relationships and molecular mechanisms linking xenobiotic exposure and gut dysbiosis to disease development [1]. These methods are also essential for the comprehensive toxicological assessment of xenobiotics, including endocrine-disrupting chemicals (EDCs), a major class of environmental contaminants with potential health risks [2]. EDCs can enter the body not only through ingestion with food and water but also via inhalation or dermal absorption. Once in the gastrointestinal tract, xenobiotics interact with the GM before being absorbed and detoxified in the liver. Some may be excreted or return to the gut via bile, allowing further interaction with the GM [3].
Xenobiotics can directly affect the taxonomic structure of the GM, leading to alterations in microbial diversity and the abundance of specific bacterial species [4]. Changes in microbial metabolite production, including short-chain fatty acids (SCFAs) and tryptophan derivatives, may impact both gut and systemic host functions, including the gut–brain axis [5–7]. Thus, xenobiotics may impact a broad range of host physiological functions through GM-mediated metabolic activity [5,8].
Gut microorganisms can directly interact with xenobiotics, affecting both their metabolism and elimination and modulating their biological activity [9]. The GM possesses a broad enzymatic repertoire capable of transforming xenobiotics into less toxic metabolites; however, in some cases, microbial metabolism may produce compounds with increased toxicity compared to the parent compound [10]. In addition to enzymatic activity, xenobiotics may also be passively inactivated by adsorption to bacterial cell walls, a process that reduces their intestinal absorption and bioavailability [11].
This protocol enables the preparation of cryopreserved inocula from pooled human fecal samples that can be used further in a wide range of toxicomicrobiomics studies focused on GM–xenobiotic interactions. By combining samples from multiple donors into a single, well-characterized inoculum and cryopreserving it, researchers can reduce inter-individual variability, improve reproducibility, and streamline comparative studies across different conditions or compounds. The resulting material can be used repeatedly, enabling long-term storage and controlled experimental design without the need for repeated donor recruitment and processing. Importantly, our cryopreservation and standardized inoculum preparation protocol preserves approximately 60% of the unique bacterial taxa and ~75% of the overall microbial community complexity in the viable fraction after thawing, ensuring sufficient representativeness for downstream applications.
However, it must be noted that ~40% of unique taxa are not recovered after thawing. A likely explanation is that many bacteria are already non-viable by the time they reach the end of the gastrointestinal tract [12]. Other contributing factors may include limitations of current handling procedures or damage caused by the freeze–thaw process. Currently, no cryopreservation method enables the complete recovery of all gut microbes. Our protocol aims to preserve and propagate the viable and metabolically active portion of the community, providing a reproducible and representative inoculum for in vitro studies within current technical limitations. The method is particularly well-suited for high-throughput screening of various compound classes that interact with the gut microbiota, including xenobiotics (e.g., pharmaceuticals, endocrine disruptors), food additives, dietary components (e.g., polyphenols, fibers), and probiotics. Our team has successfully applied this approach to investigate the impact of EDCs, such as bisphenols, on the GM [2], to evaluate the effect of different culture media compositions [13], and to explore the GM's response to biogenic amines and ice cream formulations (unpublished data). In addition to GM cultivation, this method is compatible with a broad range of downstream applications, including 16S rRNA gene sequencing, shotgun metagenomics, untargeted metabolomics, and targeted analysis of microbial metabolites, such as SCFAs. Moreover, the culture supernatants obtained using this standardized inoculum can be used to study GM–host interactions, such as the effects of microbial metabolites on epithelial cells in vitro. In our studies, we have successfully applied post-fermentation fluids to investigate the impact of GM-derived compounds on intestinal epithelial cell viability [2]. This approach provides a versatile and scalable model for exploring microbial activity under controlled and reproducible conditions.
Materials and reagents
Biological materials
1. Fecal samples; these should be collected directly into a sterile stool container filled with transport medium (see Solutions). Samples should be stored at 4 °C and processed within a defined time window (preferably within 4 h of collection).
Donors are classified as healthy based on a pre-screening questionnaire (see Supplementary Material S1). Key exclusion criteria include (i) antibiotic or probiotic use within the last 3 months, (ii) reported gastrointestinal disorders within the last 3 months, (iii) diarrhea or vomiting within the past 2 weeks, and (iv) any chronic diseases or regular medication use. All procedures involving human-derived material must be approved by the relevant Institutional Ethics Committee before collection.
Reagents
1. Ultrapure type I water, generated by a Milli-Q system or similar
2. Oxyrase for broth (Sigma-Aldrich, catalog number: SAE0013-50ML)
3. Peptone-buffered water (Oxoid, catalog number: T0000041)
4. L-cysteine hydrochloride (Merck, catalog number: 2430-100GM)
5. Resazurin sodium salt (Merck, catalog number: R7017-1G)
6. Glycerol (Merck, catalog number: G7893)
7. Resazurin (Merck, catalog number: R7017)
8. NaOH (Warchem, catalog number: 56992)
9. HCl (Warchem, catalog number: 45771)
10. Vitamine K3 (Pol-Aura, catalog number: PA-03-3614-V#25G)
11. Defibrynated sterile sheep blood (GRASO Biotech, catalog number: 1000)
12. Schaedler broth (GRASO Biotech, catalog number: 139)
13. PMAxxTM Dye, 20 mM in H2O (Biotum, catalog number: 40069-1ML)
14. Benzalkonium chloride (Merck, catalog number: 12060)
15. Ethanol 99.8% (Pol-Aura, catalog number: 113964800)
Solutions
1. Benzalkonium chloride (see Recipes)
2. Resazurin stock solution (see Recipes)
3. Vitamin K3 stock solution (see Recipes)
4. Transport medium (see Recipes)
5. Cryopreservation medium (see Recipes)
6. Culture medium (see Recipes)
Recipes
1. Benzalkonium chloride
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Benzalkonium chloride | 0.13% | 1.33 g |
| Distilled water | n/a | Fill up to 1,000 mL |
Note: Store the solution in a squeeze bottle inside the anaerobic chamber.
2. Resazurin stock solution
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Resazurin | 0.25 mg/mL | 2.5 mg |
| Distilled water | n/a | 10 mL |
Note: Sterilize the solution by filtration through a 0.45 μm sterile syringe filter and transfer it to a light-protected amber glass bottle or wrap in aluminum foil to prevent photodegradation. Store at 4 °C in the refrigerator.
3. Vitamin K3 stock solution
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Vitamin K3 | 1.5 mg/mL | 150 mg |
| Ethanol 99.8% | n/a | 10 mL |
Note: The compound dissolves slowly; to aid dissolution, vortex briefly and place the solution on a HulaMixer for about 10 min. Filter the solution through a 0.45 μm sterile syringe filter. Store in an amber glass vial at 4 °C. Protect from light.
4. Transport medium
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Peptone-buffered water | 0.8% w/v | 8 g |
| L-cysteine hydrochloride | 0.5 g/L | 0.5 g |
| Resazurin stock solution | 1 mg/L | 4 mL |
| Distilled water | n/a | Fill up to 1,000 mL |
Note: Adjust the pH to 7.0 ± 0.2 using sterile 1 M HCl or NaOH, if necessary. Dispense into heat-resistant bottles with loose caps and autoclave at 121 °C for 15 min. After autoclaving, tighten the caps while the bottles are still warm to minimize oxygen diffusion during cooling. When still warm, bring to an anaerobic chamber to deoxygenate overnight and close with bromobutyl stoppers. Store the medium at 4 °C. Under anaerobic conditions, the medium should appear colorless.
5. Cryopreservation medium
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Peptone-buffered water | 0.8% w/v | 8 g |
| L-cysteine hydrochloride | 0.5 g/L | 0.5 g |
| Resazurin stock solution | 1 mg/L | 4 mL |
| Glycerol | 20% w/v | 200 g |
| Distilled water | n/a | Fill up to 1,000 mL |
Note: Autoclave at 121 °C for 15 min. After autoclaving, tighten the caps while the bottles are still warm to minimize oxygen diffusion during cooling. When still warm, bring to an anaerobic chamber to deoxygenate overnight and close with bromobutyl stoppers. Store the medium at 4 °C. Under anaerobic conditions, the medium should appear colorless or faintly yellow (Figure 1A).

Figure 1. Materials used for stool sample processing and anaerobic preparation of reagents. (A) Cryopreservation buffer in vial: deoxygenated (left) vs. oxygenated (right); (B) Culture medium (Schaedler broth): deoxygenated and sealed with bromobutyl stopper (left) vs. oxygenated (right); (C) Stool sample collection kit including (1) insulated box, (2) GENbag airtight bag with clip, (3) stool container with spatula filled with 10 mL of transport medium, (4) GENbag anaerobic atmosphere generator, (5) flushable stool collection sheet, (6) ice pack, (7) anaerobic indicator, (8) health status declaration form with written instructions and informed consent, (9) gloves.
6. Culture medium
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Schaedler broth | n/a | 29,7 g |
| Distilled water | n/a | Fill up to 1,000 mL |
Note: Mix thoroughly until completely dissolved. Measure the pH and adjust to 7.2 ± 0.2 using 1 M HCl or 1 M NaOH, if necessary. Autoclave at 121 °C for 15 min and tightly seal the bottle immediately after to prevent re-entry of oxygen. While the medium is still warm (but not hot), place the bottle into the anaerobic chamber via the airlock. In the airlock, loosen the caps by a quarter-turn. Once inside the chamber, unscrew the caps halfway to facilitate full deoxygenation of the medium. Leave the bottle overnight (at least 4 h) to ensure proper reduction. After deoxygenation, seal the bottle tightly with a bromobutyl stopper, remove from the chamber, and store at 4 °C. Directly before use, add necessary supplements under anaerobic conditions. For Schaedler broth, this includes vitamin K3 (1.5 mg/L) and defibrinated sheep blood, final concentration 10% v/v. After supplementation, the medium should be allowed to deoxygenate again for 4 h to overnight before use. If supplements are not pre-equilibrated in the anaerobic chamber, they may introduce residual oxygen into the medium, as they typically do not contain reducing agents. In such cases, allow the supplemented medium to deoxygenate again for 4 h to overnight, depending on the volume and oxygen load of the additives.
Laboratory supplies
1. Deep-well plates (2.2 mL 96-Well Deep Well Plate, U-Bottom, Square Well, catalog number: 503002)
2. Stomacher bags (Interscience, BagFilter® Pipet, catalog number: K-1385)
3. Sterile stool container 20 mL with a spatula (Syntesys, catalog number: 313316)
4. Flushable stool sample collection sheets (NEUCA S.A., catalog number: 91609)
5. Borosilicate lyophilization glass vials, 5 mL with threaded caps (Wheaton, Vacule vial, catalog number: 651905), equipped with bromobutyl rubber stoppers (Wheaton, catalog number: 700006G)
6. 250 mL glass bottle with blue GL45 screw cap (Merck, catalog number: DWK218013651)
7. DURANTM grey bromobutyl rubber stopper, straight plug, for GL 45 laboratory bottles (DWK Life Sciences, catalog number: 292062803)
8. DURANTM open-topped screw cap, central aperture, GL45 (DWK Life Sciences, catalog number: 292271007)
9. BagTips® (Interscience, catalog number: K-1088)
10. Insulated box, 30 × 400 × 24 cm (DIZ, catalog number: ST10)
11. Icepack (Merck, mPAGE® Freezer Pack, catalog number: FP2)
12. Sterile tips with filter 0.1–10 μL, 2–200 μL, 100–1,000 μL (Th. Geyer, Labsolute, catalog numbers: 7695880, 7695884, and 7695886)
13. Microcentrifuge tubes, 2 mL with lid (BRAND, catalog number: Z628034-500EA)
14. GENbag anaer: transparent airtight bags and clips, anaerobic atmosphere generators (bioMérieux, catalog number: 97067-042)
15. Chlorine-based wipe (Medilab, Chlor-Clean Wipes, catalog number: 4817333)
16. Metal tweezers (Bionovo, catalog number: 1-1811)
17. Sterilization pouch (Bionovo, catalog number: B-0403)
18. Aluminum foil (Bionovo, catalog number: B-1051)
19. Glass petri dishes, 90 mm (Duran, catalog number: G-3264)
20. Anaerobic indicator (for anaerobic chamber: Thermo Fisher Scientific, Oxoid Resazurin Anaerobic Indicator, catalog number: 10371053; for stool collection kit: Biomeriux, Anaer indicator, catalog number: 230-096118)
21. Amber glass reagent bottles with screw cap, 20 mL (WHEATON, LAB FILE, catalog number: W224604)
22. 0.45 μm membrane filters, sterile (Satorius, Minisart, catalog number: 16533-Q)
23. Centrifuge tubes (15–50 mL), depending on culture volume (Corning, catalog numbers: CLS430790 and CLS430829)
24. 250 mL squeeze bottle (LP Italiana, LDPE squeeze bottle with scale, catalog number: L-1000)
Equipment
1. Anaerobic chamber with integrated incubator (Sheldon Manufacturing Inc., BACTRON 300, catalog number: BAA30023)
2. Gas cylinders: Nitrogen (N2) and anaerobic gas mixture (90% N2, 5% CO2, 5% H2), equipped with appropriate pressure regulators
3. Laminar flow cabinet, class BSL2 (ESCO, Streamline SC2-4E1, catalog number: 2010655)
4. Autoclave (SMS, ASL 80 MSV, catalog number: 272-ASL80MSV)
5. BagPipet (Interscience, catalog number: 251091)
6. Vortexer (IKA, Vortex 2, catalog number: 0025000258)
7. Pipette, P1000 (Eppendorf Research® plus, catalog number: 3123000063)
8. Pipette, P200 (Eppendorf Research® plus, catalog number: 3123000055)
9. Pipette, P10 (Eppendorf Research® plus, catalog number: 3123000020)
10. Laboratory shaker (IKA, MS 3 basic, catalog number: 0003617000) with plate adapter (IKA, MS 3.4 Microtiter attachment, catalog number: 0003426400)
11. pH-meter (Elmetron, model: CP-315)
12. Analytical balance (Radwag, model: WAA 210/C/1)
13. Laboratory centrifuge (Eppendorf, model: 5804, catalog number: 5804000010) with rotor for 1.5 mL (Eppendorf, Rotor FA-45-30-11, catalog number: 5804726006) and 15–50 mL tubes (Eppendorf, FA-45-30-11, catalog number: 5820715006), depending on desired culture volume
14. Ultra-pure water system (Polwater, model: DL2-100)
15. -80 °C freezer (Haier Biomedical, model: DW-86L579)
16. Water bath (37 °C) (WSL, model: LWT 2/150)
17. Racks (GenoPlast Biotech S.A., catalog number: 90-8009)
18. Tube-compatible shaker (Thermo Fisher Scientific, HulaMixerTM, catalog number: 15920D)
19. PMA-LiteTM 2.0 LED Photolysis Device (Biotum, catalog number: E90006)
Software and datasets
1. QIIME2 Amplicon (v2025.7)
2. GraphPad Prism (v9.5.1, Dotmatics)
3. RStudio (Build 446, Posit PBC), with R packages: phyloseq (v1.44.0), qiime2R (v0.99.6), and vegan (v2.6-4)
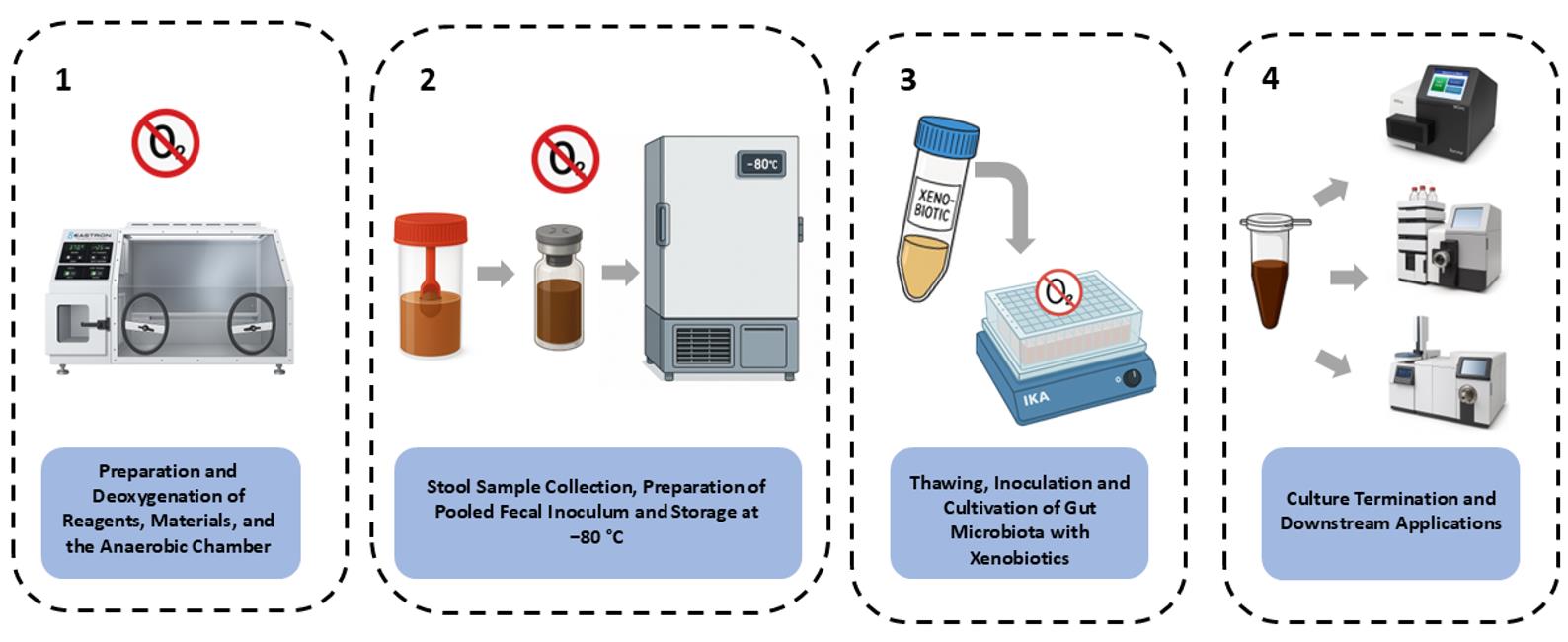
Procedure
文章信息
稿件历史记录
提交日期: Jul 29, 2025
接收日期: Sep 29, 2025
在线发布日期: Oct 17, 2025
出版日期: Nov 5, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Średnicka, P., Emanowicz, P. and Wójcicki, M. (2025). Optimized Protocol for the Collection, Cryopreservation, and In Vitro Cultivation of Human Gut Microbiota for Toxicomicrobiomics Applications. Bio-protocol 15(21): e5498. DOI: 10.21769/BioProtoc.5498.
分类
微生物学 > 群落分析 > 悉生培养系统
生物科学 > 微生物学 > 微生物群落 > 微生物组学
系统生物学 > 微生物组学
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link