- EN - English
- CN - 中文
In Silico Prediction and In Vitro Validation of Bacterial Interactions in the Plant Rhizosphere Using a Synthetic Bacterial Community
基于合成细菌群落的植物根际细菌相互作用的计算预测与体外验证
发布: 2025年11月05日第15卷第21期 DOI: 10.21769/BioProtoc.5496 浏览次数: 1649
评审: Shweta PanchalPayton Tung-On YauAnonymous reviewer(s)

相关实验方案
细菌病原体介导的宿主向溶酶体运输的抑制:基于荧光显微镜的DQ-Red BSA分析
Mădălina Mocăniță [...] Vanessa M. D'Costa
2024年03月05日 2893 阅读
通过制备连续聚丙烯酰胺凝胶电泳和凝胶酶谱分析法纯化来自梭状龋齿螺旋体的天然Dentilisin复合物及其功能分析
Pachiyappan Kamarajan [...] Yvonne L. Kapila
2024年04月05日 2080 阅读
Abstract
The rhizosphere, a 2–10 mm region surrounding the root surface, is colonized by numerous microorganisms, known as the rhizosphere microbiome. These microorganisms interact with each other, leading to emergent properties that affect plant fitness. Mapping these interactions is crucial to understanding microbial ecology in the rhizosphere and predicting and manipulating plant health. However, current methods do not capture the chemistry of the rhizosphere environment, and common plant–microbe interaction study setups do not map bacterial interactions in this niche. Additionally, studying bacterial interactions may require the creation of transgenic bacterial lines with markers for antibiotic resistance/fluorescent probes and even isotope labeling. Here, we describe a protocol for both in silico prediction and in vitro validation of bacterial interactions that closely recapitulate the major chemical constituents of the rhizosphere environment using a widely used Murashige & Skoog (MS)-based gnotobiotic plant growth system. We use the auto-fluorescent Pseudomonas, abundantly found in the rhizosphere, to estimate their interactions with other strains, thereby avoiding the need for the creation of transgenic bacterial strains. By combining artificial root exudate medium, plant cultivation medium, and a synthetic bacterial community (SynCom), we first simulate their interactions using genome-scale metabolic models (GSMMs) and then validate these interactions in vitro, using growth assays. We show that the GSMM-predicted interaction scores correlate moderately, yet significantly, with their in vitro validation. Given the complexity of interactions among rhizosphere microbiome members, this reproducible and efficient protocol will allow confident mapping of interactions of fluorescent Pseudomonas with other bacterial strains within the rhizosphere microbiome.
Key features
• This method builds upon the widely used MS-based gnotobiotic system for growing plants and a synthetic bacterial community (SynCom) for plant–microbe interaction studies.
• It considers the chemical composition of plant growth media (MS) and root exudates to map bacterial interactions.
• It provides a method to both predict and validate interactions of fluorescent Pseudomonas with other strains within a SynCom.
• This method is scalable for any bacterial pair with distinguishing markers (e.g., fluorescence, antibiotic resistance).
Keywords: Microbial interactions (微生物互作)Graphical overview
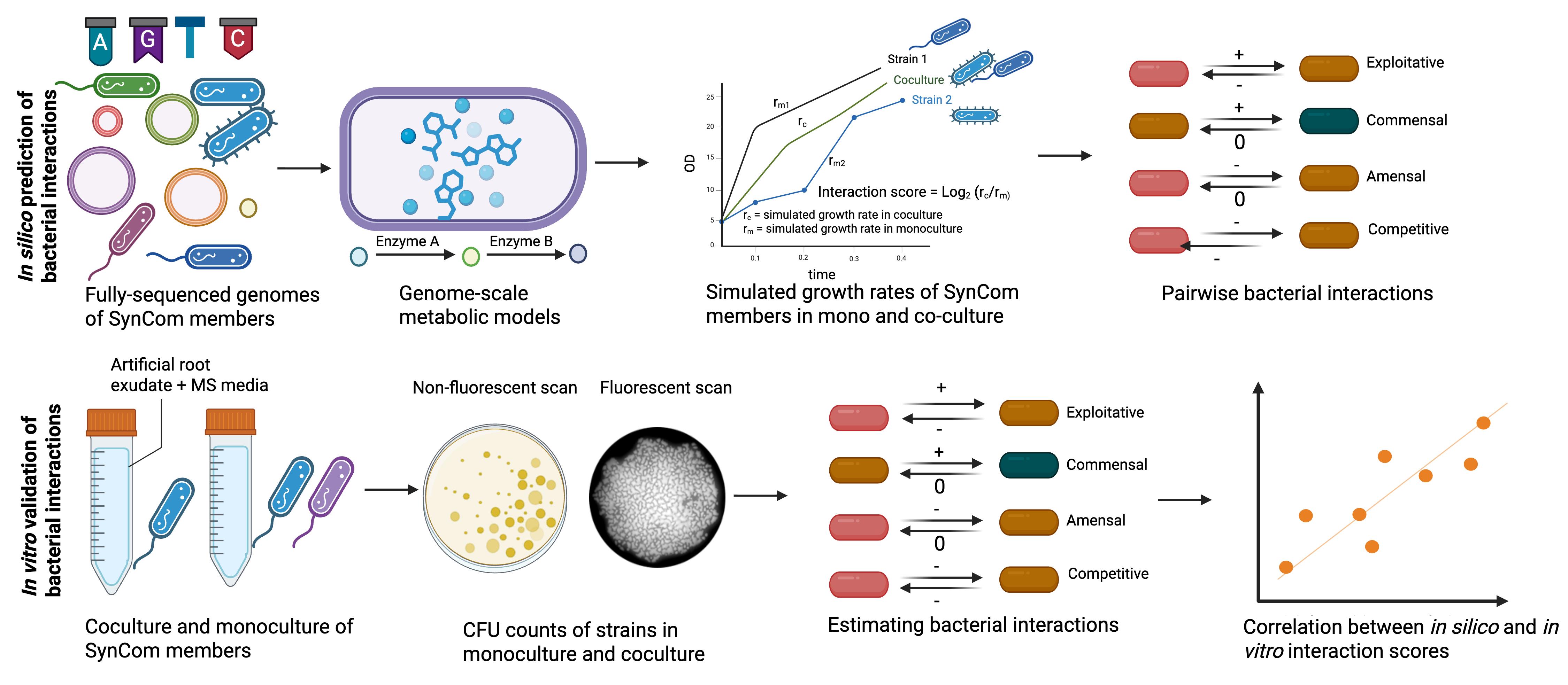
Workflow for in silico prediction and in vitro validation of bacterial interactions. Genome sequences of synthetic bacterial community (SynCom) members are used to infer their genome-scale metabolic models (GSMMs). These models are then used to simulate growth in monoculture and in coculture with the interacting strains in chemically defined artificial root exudates + MS media. This same media is later used to calculate interaction scores and classify them in ecological terms through colony-forming unit (CFU) counts, in vitro. Individual strains are grown (same media as we used for in silico prediction) in monoculture and in coculture with interacting strains for 24 h, with an initial OD of 0.02 for each of the strains. Following their growth, cultures are serially diluted in King’s B agar media to estimate the count of fluorescent Pseudomonas and the other strain using fluorescent and non-fluorescent scans. CFU counts are estimated with ImageJ and subsequently used to calculate their interaction scores. In this protocol, the fluorescent Pseudomonas sp. 6A2 is used as an example to estimate its interaction with other members of the SynCom. Any other marker, e.g., antibiotic resistance, could be used for estimating interaction scores.
Background
Plants host phylogenetically diverse microbial communities (microbiomes) across their niches—the root, shoot, and rhizosphere [1,2]. Members of the microbiome can influence plant fitness, either individually or through their interaction with other microbial members, leading to emergent properties [3]. Mapping these interactions is therefore important but remains a challenge. The introduction of synthetic bacterial communities has greatly simplified our ability to deconstruct these complex microbe–microbe interactions.
Pairwise bacterial interactions could be mapped experimentally and have been shown to infer complex ecological dynamics within multi-species communities [4,5]. In parallel, advances in computational approaches have greatly enhanced our ability to predict these interactions. Using genome-scale metabolic models (GSMMs), researchers have mapped the microbial interactions in diverse ecosystems, including the human gut and the phyllosphere [6,7]. This approach is more accurate than correlation-based approaches, enabling the prediction of numerous possible interactions within a microbial community, which otherwise is tedious to perform experimentally [8]. However, it is crucial to validate them experimentally for better confidence in these computational predictions.
Plate-based systems are traditionally used to study plant–microbe interactions in a gnotobiotic setup [9]. Microbes added to the plant roots rely on root exudates for survival and colonization. Additionally, the chemistry of the root exudates could greatly influence bacterial interactions in the rhizosphere. The chemical environment in these systems greatly influence the outcome of plant–microbe and microbe–microbe interactions. Although a few studies have attempted to map bacterial interactions within the plant microbiome, the chemical environment of Murashige & Skoog (MS)-based gnotobiotic systems and the effects of plant-derived chemicals are often ignored [10]. In this protocol, we present both computational and experimental validation of bacterial interactions, considering the chemistry of root exudates and the MS-based gnotobiotic system. We use a fluorescent pseudomonad species within our synthetic bacterial community (SynCom) to differentiate its colony-forming unit (CFU) counts from other bacterial species in coculture. Since fluorescent pseudomonads are a majorly abundant bacterial species in the rhizosphere, this protocol will be useful in future studies [11]. Even though the chemical composition of root exudates is simplified, our computational prediction and experimental validation correlate with each other. This method for mapping bacterial interactions in the rhizosphere can be extended to other gnotobiotic plant-growth systems and plant niches.
Materials and reagents
Biological materials
1. The following 17 bacterial strains were used to study their interactions with the fluorescent strain Pseudomonas sp. 6A2: (i) Paenibacillus sp. 8E4, (ii) Pseudomonas sp. 9F3, (iii) Mycoplana sp. P31D, (iv) Brevundimonas sp. 9B2, (v) Candidimonas sp. 4C, (vi) Achromobacter sp. 1A1, (vii) Xanthomonas sp. 3C2, (viii) Atlantibacter sp. P33G, (ix) Lysinibacillus sp. 3C1, (x) Bhargaveae sp. 9B1, (xi) Bacillus sp. 8A2, (xii) Priestia sp. 7F21, (xiii) Corynebacterium sp. 10B2, (xiv) Agromyces sp. 9E2, (xv) Cellulosimicrobium sp. 8E1, (xvi) Streptomyces sp. P32B1, (xvii) Streptomyces sp. 6A1
In this protocol, this collection of 17 strains is named SynCom18.
All the above strains can be grown to saturation within 24 h in Luria broth (LB) media. Store the strains in 25% glycerol at -80 °C for long-term storage. While growing the strains for monoculture/coculture experiments, streak them as single colonies in LB agar plates.
Note: The inherent fluorescence of Pseudomonas sp. 6A2 is used as a distinguishing feature for CFU counts of individual strains in coculture. This method, therefore, requires distinguishing markers of bacterial strains to estimate CFU in coculture with other bacterial strains. This could be colony morphology, fluorescence, or antibiotic resistance.
Reagents
1. Glucose (Sigma, CAS number: 50-99-7)
2. Fructose (Sigma, CAS number: 57-48-7)
3. Sucrose (Sigma, CAS number: 57-50-1)
4. Succinic acid (Sigma, CAS number: 110-15-6)
5. L-Alanine (Sigma, CAS number: 56-41-7)
6. L-Serine (Sigma, CAS number: 56-45-1)
7. Citric acid (Sigma, CAS number: 77-92-9)
8. Sodium lactate (Sigma, CAS number: 72-17-3)
9. Glycine (Sigma, CAS number: 56-40-6)
10. Nicotinic acid (Sigma, CAS number: 59-67-6)
11. Pyridoxine HCl (Sigma, CAS number: 58-56-0)
12. Thiamine HCl (Sigma, CAS number: 67-03-9)
13. MS (Sigma, catalog number: M5519)
14. MES hydrate (Sigma, CAS number: 1266615-59-1)
15. Glycerol (Sigma, CAS number: 56-81-5)
16. Luria broth (Sigma, catalog number: 146813)
17. Bacto agar (Sigma, CAS number: 9002-18-0)
18. King agar B (Sigma, catalog number: 60786)
19. Magnesium chloride (MgCl2) (Sigma, catalog number: M8266)
Solutions
1. Artificial root exudates (ARE), 10× stock (see Recipes)
2. Vitamin stock (1,000×) (see Recipes)
3. MS media (2× stock) (see Recipes)
4. Luria broth (LB)
Recipes
Note: Prepare all solutions in a sterile laminar hood and store in sterile glass bottles.
1. Artificial root exudates (ARE), 10× stock
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Glucose | 16.4 g/L | 100 mL |
| Fructose | 16.4 g/L | 100 mL |
| Sucrose | 8.4 g/L | 100 mL |
| Succinic acid | 9.2 g/L | 10 mL |
| Alanine | 8 g/L | 10 mL |
| Serine | 9.6 g/L | 10 mL |
| Citric acid | 3.2 g/L | 10 mL |
| Sodium lactate | 6.4 g/L | 10 mL |
ARE recipe was adapted from Getzke et al. [12].
2. Vitamin stock (1,000×)
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Glycine | 2,000 mg/L | 10 mL |
| Nicotinic acid | 500 mg/L | 10 mL |
| Pyridoxine HCl | 500 mg/L | 10 mL |
| Thiamine HCl | 100 mg/L | 10 mL |
Can be stored at 4 °C for approximately 1 month.
3. MS media (2× stock)
Prepare a 2× stock of MS media by adding 8.8 g of MS salt in 1 L of distilled water with 1 g of MES hydrate. MS media can be stored at 4 °C for approximately 1 month after autoclaving.
Prepare 100 mL of ARE + vitamin stock + MS media by adding 50 mL of 2× MS stock to 10 mL of ARE 10× stock and 100 μL of 1,000× vitamin stock. Filter-sterilize ARE and vitamin stock using a 0.22 μm membrane before adding to MS media. Autoclave (121 °C for 20 min) and cool down the MS media before adding to ARE and vitamin solution. Set the pH of the total mixed solution to 7 before use.
Laboratory supplies
1. 50 and 15 mL Falcon tubes (NuncTM Conical Sterile Polypropylene Centrifuge Tubes) (Thermo Scientific, catalog number: 12-565-269)
2. 1.5 and 2 mL tubes (Safe-Lock Tubes 1.5 mL, Microtube) (Eppendorf, catalog number: 05-402-27)
3. L-shaped Spreader (L-Shape, sterile) (Sigma, catalog number: HS8171B)
4. Petri plates (NuncTM Petri dishes) (Thermo Scientific, catalog number: 08-757-099)
5. Inoculation loops (Thermo Scientific, Disposable Calibrated Inoculating Loops, catalog number: R501400)
6. Pipette tips (1–10 μL, 20–200 μL, and 100–1,000 μL) (FinntipTM Pipette Tips, catalog number: 9401115)
7. Pipettes (1–10 μL, 20–200 μL, and 100–1,000 μL) (PIPETMAN 4-Pipette Kit, catalog number: F167360)
Equipment
1. Plate reader (Agilent BioTek Synergy H1 Multimode Reader)
2. Gel scanner 2 (GelDoc Go Gel Imaging System with Image Lab Touch Software, catalog number: 12009077)
Software and datasets
1. Image J (v1.54p freely available) [13]
2. R (v4.4.1 freely available)
3. Python (v3.8 freely available)
4. MEMOTE (v0.14.0) (python-based application and freely available) [14]
5. gapseq (v1.4.0) (R-based application and freely available) [15]
All data and code have been deposited to GitHub: https://github.com/arijitnus/Bio-protocol/ (access date, 05/16/2025).
Procedure
文章信息
稿件历史记录
提交日期: May 21, 2025
接收日期: Sep 25, 2025
在线发布日期: Oct 15, 2025
出版日期: Nov 5, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Mukherjee, A., Tan, B. H. and Swarup, S. (2025). In Silico Prediction and In Vitro Validation of Bacterial Interactions in the Plant Rhizosphere Using a Synthetic Bacterial Community. Bio-protocol 15(21): e5496. DOI: 10.21769/BioProtoc.5496.
分类
生物信息学与计算生物学
微生物学 > 微生物-宿主相互作用 > 细菌
植物科学 > 植物免疫 > 宿主-细菌相互作用
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link