- EN - English
- CN - 中文
A Quantitative Spectrophotometric Assay Matched With Environmental Scanning Electron Microscopy to Measure Calcium Crystals in Human Osteoarthritic Synovial Fluid
结合环境扫描电子显微镜的定量分光光度法检测人骨关节炎滑液中的钙晶体
发布: 2025年11月05日第15卷第21期 DOI: 10.21769/BioProtoc.5495 浏览次数: 1240
评审: Olga KopachAnonymous reviewer(s)
Abstract
In the field of osteoarthritis (OA), the identification of reliable diagnostic and prognostic biomarkers in patients with hip lesions such as femoroacetabular impingement (FAI) could have an immeasurable value. Calcium crystal detection in synovial fluids (SFs) is one tool currently available to diagnose patients with rheumatologic disorders. Crystals, such as monosodium urate (MSU) and calcium pyrophosphate (CPP), are identified qualitatively by compensated polarized light, whereas basic calcium phosphate (BCP) crystals are visualized under conventional light microscopy by Alizarin red S (ARS) staining. Here, we present an efficient and straightforward protocol to quantify calcium crystals by spectrophotometric analysis in human osteoarthritic SFs after staining with ARS. The type and size of the different crystal species are confirmed by environmental scanning electron microscopy (ESEM).
Key features
• This protocol provides a quantitative assay to measure calcium crystals in human synovial fluids.
• ARS is specific for hydroxyapatite, calcium phosphate, tetrasodium pyrophosphate, sodium phosphate, calcium chloride/pyrophosphate dihydrate, and oxalate crystals; does not show any combination with SFs components.
• The standard curve for crystal quantification was prepared with a synthetic hydroxyapatite, which allows to prepare a series of stable and reproducible standards.
• The analysis with ESEM determines the elemental composition of all visible particles without any pretreatment of the biological sample prior to observation.
Keywords: Biomarker (生物标志物)Graphical overview
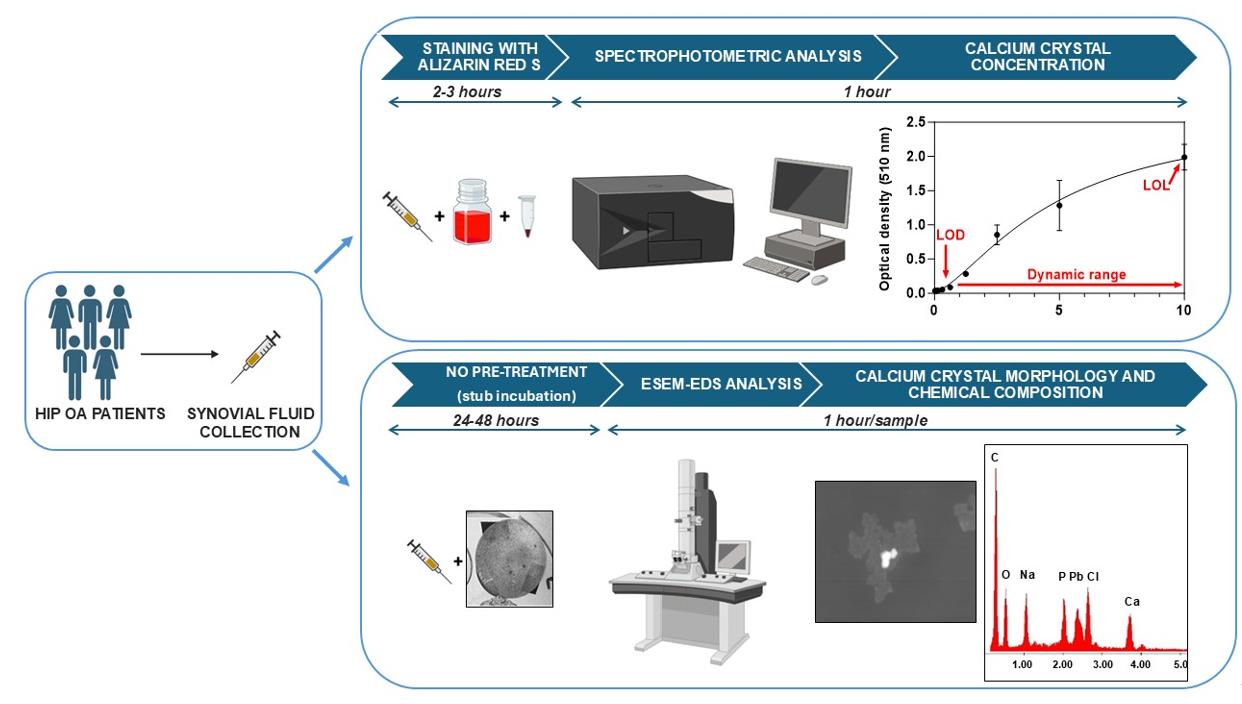
Overview of the calcium crystal quantification by spectrophotometric analysis and identification by environmental scanning electron microscope equipped with an energy dispersive spectrometer (ESEM-EDS)
Background
Biomarkers are biological molecules present in body fluids or tissues that may be used as indicators of physiological and pathophysiological processes. Moreover, they can be used to see how well patients respond to new treatments and interventions for a disease or condition [1]. In the field of osteoarthritis (OA), biomarkers of early OA represent a major unmet need, and more research is required to identify biomarkers that characterize early events in the pathogenesis of OA [2]. The identification of reliable diagnostic and prognostic biomarkers might help guide decision making in an efficient and cost-effective manner. In particular, the early recognition and management of patients with hip lesions, such as femoroacetabular impingement (FAI), may preempt significant hip morbidity as OA [3–5].
Calcium crystal detection in synovial fluid (SF) is one tool currently available to diagnose patients with rheumatologic disorders, associated with biochemical markers and advanced imaging [6]. Routinely, crystals such as monosodium urate (MSU) and calcium pyrophosphate (CPP) are identified qualitatively by compensated polarized light, whereas positivity for basic calcium phosphate (BCP) crystals is visualized under conventional light microscopy by Alizarin red S (ARS) staining [7]. There is a big limitation in the visualization under conventional light microscopy of the calcium crystals stained with ARS, related to the presence of crystal clumps in the SF. Therefore, interpreting BCP qualitative detection is challenging as ARS-stained crystal clumps may resemble apatite, and small CPP crystals might be missed under polarized light.
The detection of calcium crystals in synovial fluid can be performed using an environmental scanning electron microscope (ESEM) [8]. ESEM technology represents an upgrade of the conventional scanning electron microscope (SEM). Indeed, ESEM allows the observation of samples, even at high resolution and without any conductive coating on the sample, at different vacuum levels. This type of opportunity is particularly useful for biological samples, as it allows morphological analysis without any pretreatment prior to observation. In addition, when equipped with an energy dispersive spectrometer (EDS), the ESEM allows the semiquantitative detection of the chemical elements that constitute the ultrastructural components of the sample (point or areal analysis), with the same high spatial resolution as morphological analysis and without interference related to sample preparation (chemical stabilization, dehydration, and conductive coating), as using a conventional SEM.
Our protocol suggests a quantitative method to measure calcium crystals in human OA SF associated with ESEM to identify and measure the size of the different crystal species.
Materials and reagents
Biological materials
1. Synovial fluid samples were collected from five patients with late hip OA, assessed by the Kellgren–Lawrence grade system [9], aliquoted without any pretreatment and stored at -80 °C for longer storage
Reagents
1. Synthetic hydroxyapatite (HAP) (Bioteck, catalog number: HAP038C5SD09C)
2. Alizarin Red S (Merck, catalog number: A5533)
3. Ultrapure distilled water DNase/RNase-free (Invitrogen, catalog number: 10977-035)
4. Acetic acid glacial (Carlo Erba Reagents srl, catalog number: 401422)
5. Ammonium hydroxide solution, 28.0%–30.0% NH3 basis (Sigma-Aldrich, catalog number: 338818)
6. Phosphate buffer saline (PBS) w/o calcium w/o magnesium (BioNORDICA, catalog number: EC-ECB5004L)
Solutions
1. Alizarin Red S (ARS) solution (see Recipes)
2. 10% acetic acid (see Recipes)
3. 0.15% ammonium hydroxide (see Recipes)
4. 3% ammonium hydroxide (see Recipes)
5. Synthetic hydroxyapatite (HAP) stock solution (see Recipes)
Recipes
1. Alizarin Red S (ARS) solution
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Alizarin Red S (powder) | 40 mM | 0.548 g |
| Distilled water | 40 mL |
a. Weigh 0.548 g of ARS in a beaker with an analytical balance and add 30 mL of distilled water with a 25 mL pipette.
b. Mix at room temperature (RT) in a magnetic stirrer until completely dissolved.
c. Adjust the solution to pH 4.2 by adding 0.15% of ammonium hydroxide drop by drop while stirring using an electrode pH meter.
d. Transfer the solution to a graduated cylinder and add distilled water up to 40 mL of final volume.
e. Filter the solution with a 0.45 µm microfilter and store in a dark bottle at RT for a maximum of one month.
f. Filter the solution with a 0.22 µm microfilter before use.
2. 10% acetic acid
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Acetic acid glacial (liquid) | 10% | 10 mL |
| Distilled water | 90 mL |
a. Add 10 mL of acetic acid glacial to 90 mL of distilled water to make 100 mL of solution in a graduated cylinder.
b. Mix at RT in a magnetic stirrer until completely mixed and transfer the solution into a bottle to store at RT for a maximum of six months.
3. 0.15% ammonium hydroxide
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Ammonium hydroxide (liquid) | 0.15% | 500 μL |
| Distilled water | 99.5 mL |
a. Add 500 μL of ammonium hydroxide commercial solution (28.0%–30.0% NH3 basis) and distilled water up to 100 mL of final volume in a graduated cylinder.
b. Mix at RT in a magnetic stirrer until completely mixed and transfer the solution into a bottle to store at RT for a maximum of six months.
c. Check the presence of possible precipitates and gently mix before use.
4. 3% ammonium hydroxide
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Ammonium hydroxide (liquid) | 3% | 1 mL |
| Distilled water | 10 mL |
a. Prepare fresh before use.
b. Add 1 mL of ammonium hydroxide commercial solution (28.0%–30.0% NH3 basis) to 9 mL of distilled water in a 15 mL centrifuge tube.
c. Swirl the tube to mix.
5. Synthetic hydroxyapatite (HAP) stock solution
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| HAP (powder) | 10 mg/mL | 10 mg |
| PBS | 1 mL |
a. Prepare fresh before use.
b. Weigh 10 mg of HAP powder with an analytical balance in a 1.5 mL conical vial and mix with 1 mL of PBS.
c. Vortex the vial to mix.
Laboratory supplies
1. Safe-lock 1.5 mL conical vial (Eppendorf, catalog number: 0030120086)
2. Serological pipettes 5, 10, 25 mL (Sarsted, catalog numbers: 86.1253.001, 86.1254.001, 86.1685.001)
3. Pipette tips epTIPS 0.1–20 μL and 50–1,000 μL (Eppendorf, catalog numbers: 022492012 and 0030076176)
4. Pipette tips FINNTIP 250 universal (Thermofisher Scientific, catalog number: 9400230)
5. Sterile syringe filters MINISART NML 0.22 and 0.45 micron (Sartorius, catalog numbers: S6534 and S6555 FMGUK)
6. 20 mL syringe (Caress, catalog number: CCAR02003000)
7. 15 mL centrifuge tube (Euroclone, Primo EZ tubes 15 mL, catalog number: ET5015B)
8. 96-well, non-treated, flat-bottom microplate (Thermofisher Scientific, NUNC, catalog number: 267427)
9. Aluminum stub covered with a conductive carbon adhesive disk (TAAB Ltd., catalog number: C263/N)
Equipment
1. Refrigerated microcentrifuge (DuPont, model: RMC-14)
2. Analytical balance (Mettler Toledo, model: 303-S/FACT)
3. pH meter (CRISON, model: GLP21)
4. Maxi mixer vortex (SECURLAB, model: 714)
5. Microplate spectrophotometer (TECAN Trading AG, model: NanoQuant Infinite M200)
6. Chemical hood (BICASA SRL, model: Lite 120)
7. Environmental scanning electron microscope (FEI, model: Quanta 200 FEG) equipped with an energy-dispersive X-ray spectrometer with an ECON (Edax Carbon Oxigen Nitrogen) 6 utw X-ray detector (EDAX Inc.)
Software and datasets
1. i-control 1.10 (for infinite reader) (TECAN, Version 1.10)
2. GraphPad Prism (GraphPad Software Boston, MA, v. 10.0.2)
3. Genesis Spectrum (EDAX Inc., Version 4.61)
4. Adobe Photoshop CS2 (ADOBE, Version 9.0)
5. Image J (Microsoft Java, Version 1.54k)
Procedure
文章信息
稿件历史记录
提交日期: Jun 27, 2025
接收日期: Sep 24, 2025
在线发布日期: Oct 17, 2025
出版日期: Nov 5, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Battistelli, M., Valentini, L. and Olivotto, E. (2025). A Quantitative Spectrophotometric Assay Matched With Environmental Scanning Electron Microscopy to Measure Calcium Crystals in Human Osteoarthritic Synovial Fluid. Bio-protocol 15(21): e5495. DOI: 10.21769/BioProtoc.5495.
分类
医学 > 发炎
生物化学 > 其它化合物
生物物理学 > 显微技术 > 扫描电子显微镜
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link








