- EN - English
- CN - 中文
Production of Genetically Engineered Extracellular Vesicles for Targeted Protein Delivery
制备基因工程化细胞外囊泡用于靶向蛋白递送
发布: 2025年11月05日第15卷第21期 DOI: 10.21769/BioProtoc.5494 浏览次数: 1644
评审: David PaulAnonymous reviewer(s)

相关实验方案

外周血中细胞外囊泡的分离与分析方法:红细胞、内皮细胞及血小板来源的细胞外囊泡
Bhawani Yasassri Alvitigala [...] Lallindra Viranjan Gooneratne
2025年11月05日 1464 阅读
Abstract
Extracellular vesicles (EVs) have emerged as promising carriers for the targeted delivery of therapeutic proteins to specific cells. Previously, we demonstrated that genetically engineered EVs can be used for targeted protein delivery. This protocol details the generation of mannose receptor (CD206)-targeted EVs using a modular plasmid system optimized for production in HEK293T cells. Three plasmids enable customizable EV budding, cargo loading, and surface modification for targeting to antigen-presenting cells (APCs). EVs are isolated via differential centrifugation and chromatography, characterized using transmission electron microscopy (TEM) and nanoparticle tracking analysis (NTA), and validated through functional uptake assays in primary human activated dendritic cells. Our approach combines flexibility in engineering required EVs with robust, reproducible isolation and characterization workflows. Its modularity allows easy adaptation to alternative targets or cargoes, which can be validated immediately through in vitro testing.
Key features
• First detailed protocol for generating genetically engineered EVs with fusogenic VSV-G protein and CD206-specific targeting.
• Enables rapid customization of EVs for diverse therapeutic cargo and cell-targeting applications.
• Integrates gold-standard EV isolation with multi-modal characterization to ensure reliability.
• A universal platform for customizable cell targeting: swapping VSV-G-linked llama nanobodies with diverse specificities.
Keywords: EVs (细胞外囊泡)Graphical overview
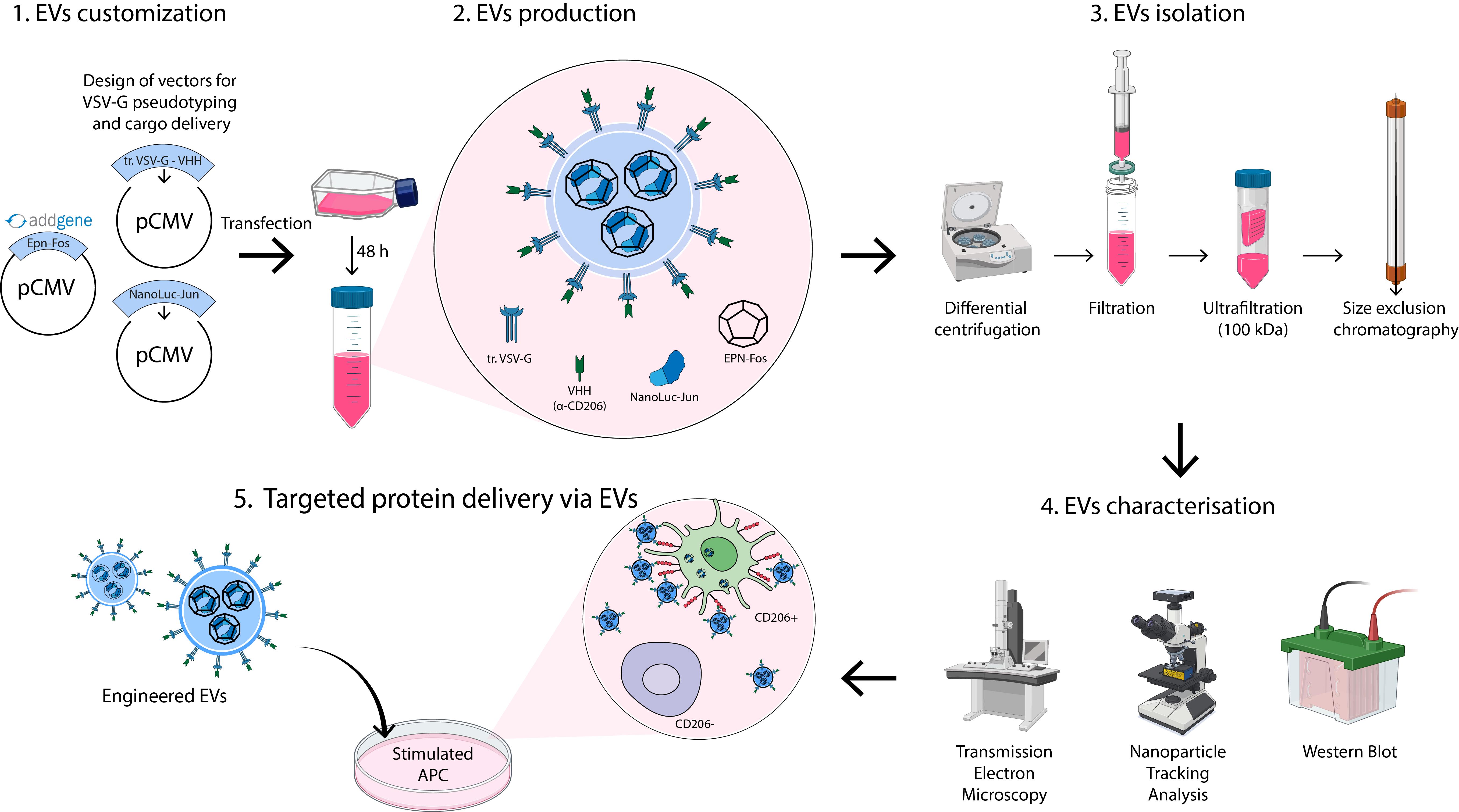
Background
Targeted delivery of therapeutic proteins to specific cells represents a promising strategy for treating a variety of autoimmune, cancer, and viral diseases. To maximize efficacy and safety, numerous delivery platforms are being developed, with the primary goals of widening the therapeutic window, minimizing off-target effects, and overcoming challenges related to the solubility and stability of bioactive molecules. Among the diverse range of carrier systems under investigation, extracellular vesicles (EVs) have emerged as particularly promising candidates due to their inherent biocompatibility and modularity [1,2].
A key advantage of EVs lies in their naturally derived, biodegradable lipid bilayer, which can be engineered to modulate protein composition, physicochemical properties, and surface markers for cell-specific targeting [3,4]. A prominent example of such engineering is the use of the vesicular stomatitis virus glycoprotein G (VSV-G). VSV-G-pseudotyped EVs have been demonstrated to mediate efficient intracellular protein delivery in vitro and facilitate genome-editing processes in vivo [5]. Parallel to the bioengineering of natural EVs, advances in protein engineering have enabled the de novo design of self-assembling protein scaffolds. For instance, the KDPG aldolase from Thermotoga maritima—which naturally forms trimeric building blocks—was engineered to assemble into hyperstable dodecahedron structures—enveloped protein nanocages (EPNs) [6]. A pivotal step toward integrating these fields was taken by Votteler et al., who designed a hybrid platform employing VSV-G-pseudotyped EVs to encapsulate such synthetic protein scaffolds [7]. The resulting EVs, which feature a protein dodecahedron nanocage within a lipid bilayer, are capable of efficient autonomous release from producer cells.
Accurate and efficient isolation of EVs is a critical step in EV research, yet it remains a significant challenge [8]. Currently, no universal isolation method exists; the choice of technique depends on the study’s specific requirements, including yield, purity, and downstream applications [9]. To enhance both recovery and purity, researchers often combine multiple methods. While novel EV isolation techniques are continually emerging, well-established protocols—such as differential centrifugation followed by ultracentrifugation and size-exclusion chromatography (SEC)—remain widely used due to their reproducibility and reliability [10–12].
Following isolation, EV characterization is essential to confirm identity, purity, and functionality. Imaging techniques play a pivotal role, with transmission electron microscopy (TEM), scanning electron microscopy (SEM), and cryo-electron microscopy (cryo-EM) being the most common [13]. Notably, TEM often produces cup-shaped EV images due to sample dehydration and staining, whereas cryo-EM preserves EVs’ native morphology, providing more accurate structural insights [14]. For size distribution analysis, dynamic light scattering (DLS) is frequently employed [15]. This non-invasive technique measures fluctuations in scattered light caused by Brownian motion to determine the hydrodynamic diameter and polydispersity index (PDI). However, while DLS is suitable for monodisperse particles in solution (PDI < 0.07, as per ISO 22412:2017), its accuracy is significantly compromised for heterogeneous EV samples due to inherent size polydispersity and sensitivity to larger particles/aggregates. Consequently, techniques like nanoparticle tracking analysis (NTA) or tunable resistive pulse sensing (TRPS) are strongly preferred for size profiling and concentration measurement of EVs. Both techniques operate on a particle-by-particle basis, well-suited for complex biological polydisperse samples like EVs [16]. Nevertheless, as they detect all particles within their size sensitivity range (e.g., protein aggregates or contaminants), appropriate blank controls are essential to ensure EV-specific measurements.
Beyond physical properties, molecular profiling of EVs is crucial. Mass spectrometry and western blotting are widely used to analyze EV cargo and surface markers, ensuring proper characterization per the MISEV guidelines [17]. These techniques are particularly valuable for engineered EVs, as they verify specific cargo loading.
Here, we detail an optimized protocol for producing mannose receptor (CD206)-targeting engineered EVs from HEK293T cells, adaptable to diverse producer cell lines via transient transfection [18,19]. Our protocol allows for the isolation of 1012 highly purified engineered EVs from 1 L of media. The system requires three plasmids:
1) truncated_VSV-G_VHH_pCMV: Encodes a truncated VSV-G protein fused to a nanobody (VHH) against CD206, facilitating EV budding and targeted delivery to antigen-presenting cells (APCs).
2) EPN-Fos_pCMV: Encodes a self-assembling enveloped protein nanocage (EPN), facilitating cargo loading into EVs.
3) cargo-protein-Jun_pCMV: Encodes the protein of interest fused to the Jun domain, enabling cargo loading into EPN via Fos-Jun heterodimerization. For initial experiments, we recommend using NanoLuc-Jun_pCMV (Addgene ID: 167308), encoding NanoLuc as a model cargo, followed by substitution of the NanoLuc sequence with the gene encoding your protein of interest.
The EPN-Fos_pCMV plasmid is available through Addgene (https://www.addgene.org/167306/). Additionally, precursor plasmids enabling the construction of the modular truncated_VSV-G_VHH_pCMV (https://www.addgene.org/80054/) and cargo-protein-Jun_pCMV (https://www.addgene.org/167308/) are also available. Other plasmids are available from the corresponding author upon request. Through this customization, researchers achieve tailored EV targeting (via VHH engineering) and specific protein delivery (via cargo substitution).
As a proof of concept, we detail the production of NanoLuc-loaded EVs targeted to CD206+ APCs and provide a protocol for differentiating primary human dendritic cells (DCs) to validate EV uptake and functionality.
Materials and reagents
Biological materials
1. HEK293T cell line [Russian Cell Culture Collection (RCCC), Institute of Cytology of the Russian Academy of Sciences]
Reagents
1. Advanced DMEM (Gibco, catalog number: 12491015)
2. Advanced RPMI 1640 medium (Gibco, catalog number: 12633012)
3. Ammonium chloride (Sisco Research Laboratories Pvt. Ltd., catalog number: 25103)
4. Antibiotic-antimycotic (100×) (Gibco, catalog number: 15240062)
5. Blotting-grade blocker non-fat dry milk (Bio-Rad, catalog number: 1706404XTU)
6. Bovine serum albumin (BSA) (Sigma-Aldrich, catalog number: A7030)
7. ClarityTM Western ECL substrate (Bio-Rad, catalog number: 1705060)
8. Ethylenediaminetetraacetic acid (EDTA) (PanReac Applichem, catalog number: A1103)
9. Fetal bovine serum (FBS) (Gibco, catalog number: A5670201)
10. Fetal bovine serum (FBS), exosome-depleted (Gibco, catalog number: A2720801)
11. Ficoll Paque Plus (Cytiva, catalog number: GE17-1440-03)
12. GlutaMAXTM supplement (Gibco, catalog number: 35050061)
13. Glycine (ITW Reagents, catalog number: A1067)
14. HyCloneTM characterized FBS, U.S. origin (Cytiva, catalog number: SH30071.04)
15. Laemmli buffer 4× (Bio-Rad, catalog number: 1610747)
16. LipofectamineTM 3000 transfection reagent (Invitrogen, catalog number: L3000015)
17. MEM non-essential amino acids solution (100×) (Gibco, catalog number: 11140035)
18. Nano-Glo luciferase assay (Promega, catalog number: N1110)
19. NEBuilder HiFi DNA Assembly Cloning kit (NEB, catalog number: E5520S)
20. Opti-MEMTM reduced serum medium (Gibco, catalog number: 31985070)
21. PBS pH 7.4 (10×), without calcium and magnesium (Gibco, catalog number: 70011-036)
22. PBS pH 7.4 (1×), without calcium and magnesium (Gibco, catalog number: 10010031)
23. PE anti-human CD206 (MMR) antibody (BioLegend, catalog number: 321105)
24. PierceTM BCA Protein Assay kit (Thermo Scientific, catalog number: 23225)
25. Potassium bicarbonate (Sigma-Aldrich, catalog number: 60339)
26. Proteinase K (Evrogen, catalog number: EK101)
27. Restriction endonuclease BstBI (NEB, catalog number: R0519S)
28. Restriction endonuclease MfeI-HF (NEB, catalog number: R3589S)
29. rhGM-CSF (Sci-Store, catalog number: PSG030-10)
30. rhIL-4 (Sci-Store, catalog number: PSG040-10)
31. Sodium pyruvate (100 mM) (Gibco, catalog number: 11360070)
32. T4 DNA ligase (NEB, catalog number: M0202S)
33. Tris(hydroxymethyl)aminomethane (Sigma-Aldrich, catalog number: 1.08307)
34. Trypan blue dye (Bio-Rad, catalog number: 1450013)
35. Trypsin-EDTA (0.05%), phenol red (Gibco, catalog number: 25300054)
36. Tween-20 (Servicebio, catalog number: GC204002-100ml)
37. Uranyl acetate (UA) reagent (EMS, catalog number 22400)
Solutions
1. Complete DMEM (see Recipes)
2. Complete RPMI-1640 (see Recipes)
3. PBS 0.1% BSA (see Recipes)
4. PBS-T (see Recipes)
5. Blocking buffer (see Recipes)
6. Transfer buffer (see Recipes)
7. ACK lysis buffer (see Recipes)
8. 1% uranyl acetate (see Recipes)
Recipes
1. Complete DMEM
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Advanced DMEM | 1× | 440 mL |
| FBS | 10% | 50 mL |
| GlutaMAX supplement (100×) | 1× | 5 mL |
| Antibiotic-antimycotic (100×) | 1× | 5 mL |
Note: By default, standard FBS (catalog number: A5670201) is used unless exosome-free FBS (catalog number: A2720801) is specifically indicated.
2. Complete RPMI-1640
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Advanced RPMI-1640 | 1× | 440 mL |
| FBS | 10% | 50 mL |
| GlutaMAX Supplement (100×) | 1× | 5 mL |
| Antibiotic-Antimycotic (100×) | 1× | 5 mL |
3. PBS 0.1% BSA
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| PBS (1×) (catalog number: 10010031) | 1× | 100 mL |
| BSA | 0.1% | 0.1 g |
Filter through a 0.22 μm filter.
4. PBS-T
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| PBS (1×) (catalog number: 10010031) | 1× | 999 mL |
| Tween-20 | 0.1% | 1 mL |
5. Blocking buffer
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| PBS-T | 1× | 50 mL |
| Blotting-grade blocker non-fat dry milk | 5% | 2.5 g |
6. Transfer buffer
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Tris | 68.4 mM | 8.29 g |
| Glycine | 39 mM | 2.92 g |
| Ethanol 96% | 20% | 208 mL |
| Milli-Q water | n/a | to 1 L |
7. ACK lysis buffer
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Ammonium chloride | 150 mM | 8.02 g |
| Potassium bicarbonate | 10 mM | 1 g |
| EDTA (300 mM) | 0.1 mM | 0.33 mL |
| Milli-Q water | n/a | to 1 L |
Adjust the pH to 7.2–7.4. Filter through a 0.22 μm filter. Store at 4 °C for short-term use or aliquot and freeze at -20 °C for long-term storage.
8. 1% uranyl acetate
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Uranyl acetate (UA) | 1% | 500 mg |
| Milli-Q water | n/a | to 50 mL |
Use a magnetic stirrer. Filter through a 0.22 μm filter. Protect the solution from light and store at 4 °C.
Laboratory supplies
1. Cell culture flask 25 cm2 (T25) filter cap, treated (SPL, catalog number: 70025)
2. Cell culture flask 75 cm2 (T75) filter cap, treated (SPL, catalog number: 70075)
3. Conical tubes, 15 mL (SPL, catalog number: 50015)
4. Conical tubes, 50 mL (SPL, catalog number: 50050)
5. PES membrane syringe filters, 0.45 μm (Corning, catalog number: 09-754-29)
6. Amicon Ultra-15 100 kDa centrifugal filter units (Merck, Millipore, catalog number: UFC910024)
7. Protein LoBind tubes 1.5 mL (Eppendorf, catalog number: 0030108116)
8. Superose 6 10/300 column (Cytiva, catalog number: 17517201)
9. Microplates, 384-well, PS, μCLEAR®, black, non-binding (Greiner Bio-One, catalog number: 781906)
10. Nitrocellulose western blotting membrane Amersham Protran 0.45 (Cytiva, catalog number: 10600012)
11. VACUETTE® EDTA-K2-coated tubes 9.0 mL (Greiner Bio-One, catalog number: 455045)
12. Parafilm M laboratory film (Pechiney Plastic Packaging Company, catalog number: PM996)
13. Carbon-coated TEM grids 200 mesh (EMS, catalog number: CF200-Cu-50)
14. Sharp tweezers (Ted Pella, DUMONT Biology, catalog number: 504)
15. Glass reagent bottle with GL45 PP cap and PP outlet ring, 250 or 500 mL (Simax, catalog number: 1632414321250 or 1632414321500)
16. Two-piece 2 mL plastic medical syringe with needles BD DiscarditTM II (BD, catalog number: 300928)
17. Self-standing PP centrifuge tubes 50 mL (Corning, catalog number: 430921)
Equipment
1. Laboratory ultrapure water system (Merck Millipore, model: Milli-Q IQ 7000)
2. Autoclave (Tuttnauer, model: 2840EL)
3. pH meter (Mettler Toledo, model: SevenMulti)
4. Spectrophotometer (Thermo Fisher Scientific, model: ND-2000)
5. Thermal cycler (Bio-Rad, model: T100)
6. Microcentrifuge (Eppendorf, model: 5415R) with fixed-angle rotor (Eppendorf, model: FA-45-24-11)
7. Flow cytometer (ACEA Biosciences, model: NovoCyte 2060)
8. Nanoparticle tracking analysis system (Malvern Panalytical, NanoSight Ltd, model: NanoSight LM10 HS-BF with 405 nm, 65 mW laser unit and high sensitivity camera)
9. Transmission electron microscope (JEOL, model: JEM-1400)
10. Cell sorter (Sony Biotechnology, model: SH800)
11. Glow discharge unit (Emitech Ltd., model: K100X)
12. High performance liquid chromatography (HPLC) system (Waters, model: 1525 Binary HPLC Pump with 2487 Dual λ Absorbance Detector and In-line Degasser)
13. Multimode microplate reader (Thermo Fisher Scientific, model: Varioskan LUX)
14. Gel documentation system (Bio-Rad, model: ChemiDoc MP Imaging System)
15. Centrifuge (Eppendorf, model: 5804 R) with swing-bucket rotor (Eppendorf, model: S-4-72)
Software and datasets
1. NTA Analysis Software (Malvern Panalytical, Version: NanoSight NTA 2.3 Build 0033)
2. Flow Cytometry Acquisition Software (ACEA Biosciences, Version: NovoExpress 1.6.0)
3. Particle Analysis Software (ScanEV v2.1.1)
Procedure
文章信息
稿件历史记录
提交日期: Aug 13, 2025
接收日期: Sep 26, 2025
在线发布日期: Oct 14, 2025
出版日期: Nov 5, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Ovchinnikova, L. A., Evtushenko, E. G., Bagrov, D. V., Goncharov, A. O., Gabibov, A. G. and Lomakin, Y. A. (2025). Production of Genetically Engineered Extracellular Vesicles for Targeted Protein Delivery. Bio-protocol 15(21): e5494. DOI: 10.21769/BioProtoc.5494.
分类
生物工程 > 生物医学工程 > 药物递送
细胞生物学 > 细胞器分离 > 胞外囊泡
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link










