- EN - English
- CN - 中文
Generation of 3D Human iPSC-Derived Multi-Cell Type Neurospheres for Studying Neuron, Astrocyte, and Microglia Crosstalk
构建三维人源iPSC多细胞类型神经球以研究神经元、星形胶质细胞和小胶质细胞的相互作用
(*Contributed equally to this work, §Technical contact: chrisl12@student.ubc.ca) 发布: 2025年11月05日第15卷第21期 DOI: 10.21769/BioProtoc.5493 浏览次数: 2695
评审: Sébastien GillotinAnonymous reviewer(s)
Abstract
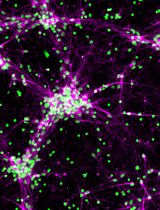
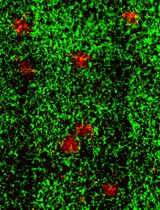
Three-dimensional (3D) human brain tissue models derived from induced pluripotent stem cells (iPSCs) have transformed the study of neural development and disease in vitro. While cerebral organoids offer high structural complexity, their large size often leads to necrotic core formation, limiting reproducibility and challenging the integration of microglia. Here, we present a detailed, reproducible protocol for generating multi-cell type 3D neurospheres that incorporate neurons, astrocytes, and optionally microglia, all derived from the same iPSCs. While neurons and astrocytes differentiate spontaneously from neural precursor cells, generated by dual SMAD-inhibition (blocking BMP and TGF-b signaling), microglia are generated in parallel and can infiltrate the mature neurosphere tissue after plating neurospheres into 48-well plates. The system supports a range of downstream applications, including functional confocal live imaging of GCaMP6f after adeno-associated virus (AAV) transduction of neurospheres or immunofluorescence staining after fixation. Our approach has been successfully implemented across multiple laboratories, demonstrating its robustness and translational potential for studying neuron–glia interactions and modeling neurodegenerative processes.
Key features
• Reproducible human iPSC-derived 3D neurosphere multi-cell type tissue culture system.
• Optional addition of microglia allows for studying neuron–microglia interaction in vitro in 3D.
• Reliable spontaneous activity offers functional tissue culture readouts of neural firing.
• System allows modeling of human brain diseases, such as Alzheimer’s disease.
Keywords: iPSC-derived cells (iPSC来源细胞)Graphical overview
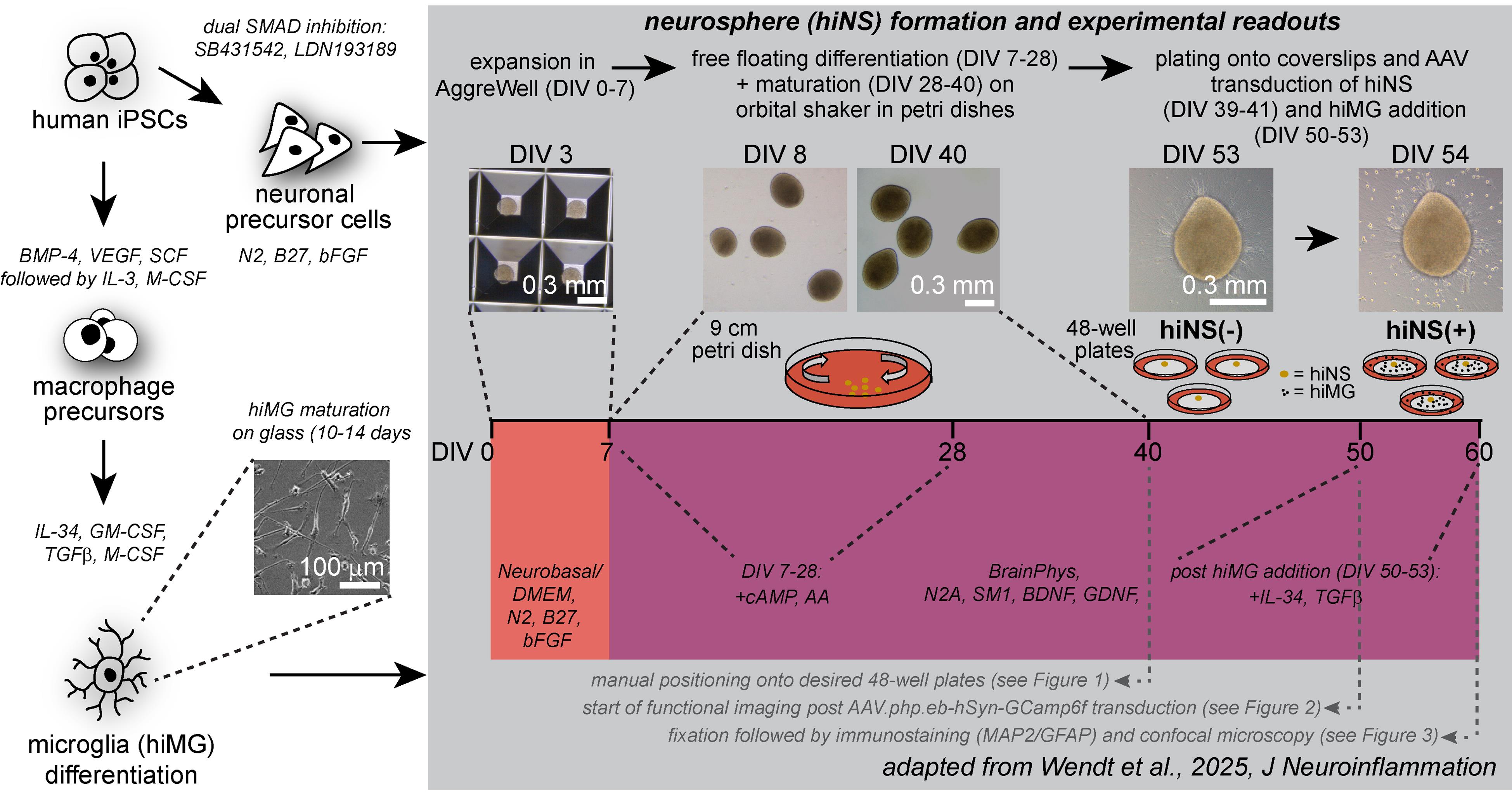
Graphical overview of human induced pluripotent stem cell (iPSC)-derived 3D neurosphere (hiNS) protocol, first presented in Wendt et al. [14]. Key steps along the protocol, media components, and experimental example readouts are highlighted. The protocol offers a reliable and robust differentiation of human 3D neural tissue comprised of neurons (both excitatory and inhibitory in nature) and astrocytes, as well as optional infiltration of microglial-like cells. This approach is particularly useful for studying microglial impact on neuron and astrocyte function in a controlled 3D tissue culture. Within the Nygaard laboratory at the University of British Columbia, this protocol was successfully used using an in house–generated iPSC line from a healthy individual as well as the commercially available KOLF2.1J [1] (The Jackson Laboratory). In addition, the Kettenmann laboratory at the Shenzhen Institutes of Advanced Technology successfully reproduced the protocol by using an additional healthy control iPSC cell line from the Chinese Academy of Sciences stem cell bank (cell line ID: DYR0100 [2])
Background
Induced pluripotent stem cells (iPSCs) were first generated by reprogramming somatic cells with defined transcription factors in mice [3], soon extended to human cells [4,5], revolutionizing personalized disease modeling. Early work focused on 2D monolayer cultures, but their limitations in mimicking tissue complexity spurred the development of 3D systems. Breakthroughs such as cerebral organoids [6] (COs) and guided cortical spheroids [7] marked the transition to iPSC-derived 3D cultures that recapitulate human brain architecture and function. Since the first report of 3D human iPSC-derived cerebral organoids for studying human mini brains in a dish [6], the organoid research field has grown and advanced substantially. COs are directly derived from iPSCs by forming ectodermal embryoid bodies followed by differentiation protocols into neural lineages. While COs can achieve remarkable tissue complexities, mimicking the embryonic human brain development [8,9], they are well known to form necrotic cores due to their large size, leading to limited oxygen and media diffusion deep within the 3D tissue [10,11]. The presence of necrotic cores is a major challenge for scientists aiming to introduce microglia, the brain resident immune cells, to CO to study their interaction with neural tissue in physiological conditions. An alternative 3D brain tissue culture approach results in the formation of smaller mini brains, largely avoiding the formation of necrotic cores: neurospheres. While neurospheres are sometimes referred to as organoids in the literature, their differentiation is distinctly different from most CO differentiation protocols. Most importantly, embryonic stem cells or iPSCs are not differentiated into embryonic bodies but rather differentiated first into neural precursor cells (NPCs) following a dual SMAD inhibition differentiation paradigm [12,13]. In suspension culture, these NPCs form aggregates that can be further differentiated, resulting into neurospheres harboring neuronal cell populations as well as astrocytes. Unlike COs, neurospheres are rapidly producible and amenable to high-throughput experimental designs, making them ideal for use in bridging the gap between mechanistic studies and translational research. We recently developed a reproducible human iPSC-derived 3D neurosphere cell culture protocol, including the addition of iPSC-derived microglia, forming a multi-cell type neural tissue, and used it to further develop a chronic amyloidosis protocol for modeling Alzheimer’s disease [14]. Here, we will provide detailed methods and step-by-step instructions for the generation of our neuron–astrocyte–microglia tri-culture neurospheres as a robust basis for studying neuron–glia interaction in physiology, or for model development for brain pathologies. Our protocol was recently reproduced at the Shenzhen Institutes of Advanced Technology; here, we show example data generated from two laboratories highlighting the reproducibility of this protocol.
Materials and reagents
Biological materials
1. HN1 human iPSC cell line (produced in-house)
2. KOLF2.1J human iPSC cell line (The Jackson Laboratory, catalog number: JIPSC001000)
3. DYR0100 human iPSC cell line (Chinese Academy of Sciences stem cell bank, number SCSP-1301)
Reagents
Note: Details are given for storage conditions. Specifics were not given over aliquot volume for supplements as this will vary depending on the number of experiments/differentiations being performed, and each research group will need to adjust volume to minimize freeze/thaw cycles.
1. mTeSRTM Plus (mTeSR Plus) (STEMCELL Technologies, catalog number: 100-0276); mTeSR Plus is provided as a base solution (400 mL; store at 4 °C) and supplement (100 mL; store at -20 °C)
2. Y-27632 (dihydrochloride) (ROCK inhibitor) (STEMCELL Technologies, catalog number: 72304); reconstitute in 1.561 mL of sterile dH2O to achieve a 10 mM solution and store as aliquots at -20 °C
3. Corning® Matrigel® growth factor reduced (GFR) basement membrane matrix, LDEV-free, 10 mL (Matrigel) (Corning, catalog number: 354230); store at -20 °C
4. PBS, pH 7.4 (ThermoFisher Scientific, catalog number: 10010023); store at 4 °C
5. Dimethyl sulfoxide, Hybri-MaxTM, sterile, suitable for hybridoma, ≥99.7% (DMSO) (Millipore Sigma, catalog number: D2650-100ML); store at RT
6. Accutase cell detachment solution (Accutase) (Millipore Sigma, catalog number: SCR005); store at 4 °C
7. ReLeSRTM (STEMCELL Technologies, catalog number: 100-0483); store at RT
8. DMEM/F-12 (ThermoFisher Scientific, catalog number: 11320033); store at 4 °C
9. NeurobasalTM medium (neurobasal) (ThermoFisher Scientific, catalog number: 21103049); store at 4 °C
10. B-27TM supplement (50×), serum free (B-27) (ThermoFisher Scientific, catalog number: 17504044); store at -20 °C
11. Cell Therapy Systems N-2 Supplement (N-2) (ThermoFisher Scientific, catalog number: 17502048); store at -20 °C
12. GlutaMAXTM supplement (GlutaMAX) (ThermoFisher Scientific, catalog number: 35050061); store at RT
13. MEM non-essential amino acids solution (100×) (MEM-NEAA) (ThermoFisher Scientific, catalog number: 11140050); store at 4 °C
14. Insulin-Transferrin-Selenium-Sodium Pyruvate (100×) (ITS-A) (ThermoFisher Scientific, catalog number: 51300044); store at 4 °C
15. 2-Mercaptoethanol (ThermoFisher Scientific, catalog number: 21985023); store at 4 °C
16. Penicillin-Streptomycin (10,000 U/mL) (Pen/Strep) (ThermoFisher Scientific, catalog number: 15140122); store at 4 °C
17. SB 431542 (Activin/BMP/TGF-β pathway inhibitor) (Tocris, catalog number: 1614)
Critical: It is required to check the lot number through tocris.com to confirm batch molecular weight and calculate dilution.
Reconstitute in DMSO to a concentration of 10 mM; store as aliquots at -20 °C.
18. LDN 193189 (Activin/BMP/TGF- β pathway inhibitor) (Tocris, catalog number: 6053)
Critical: It is required to check the lot number through tocris.com to confirm batch molecular weight and calculate dilution.
Reconstitute through two serial dilutions. First, reconstitute in DMSO to achieve a concentration of 25 mM stock solution. Further dilute 20 μL of the 25 mM stock solution with 980 μL of DMSO to achieve a concentration of 0.5 mM. Store aliquots at -20 °C.
19. Fibroblast growth factor-basic, human (bFGF) (Millipore Sigma, catalog number: F0291-25UG); reconstitute in 1 mL of sterile DH2O + 0.1% BSA to reach 25 mg/mL; store aliquots at -20 °C
20. Anti-adherence rinsing solution (STEMCELL Technologies, catalog number: 07010); store at RT
21. BrainPhysTM Neuronal Medium N2-A & SM1 Kit (BrainPhys, SM1, N2-A) (STEMCELL Technologies, catalog number 05793); note that the kit comes with two supplements; store BrainPhys at 4 °C, and SM1 and N2-A at -20 °C
22. N6,2′-O-Dibutyryladenosine 3′,5′-cyclic monophosphate sodium salt (cAMP) (Millipore Sigma, catalog number: D0627-1G); reconstitute in 20 mL of dH2O to achieve a 50 mg/mL solution, and store aliquots at -20 °C
23. Ascorbic acid, 500 mg (STEMCELL Technologies, catalog number: 72132); reconstitute through two serial dilutions. First, reconstitute in 28.4 mL of H2O to achieve a concentration of 100 mM stock solution. Further dilute 10 μL of the 25 mM stock solution with 9.98 mL of H2O to achieve a concentration of 0.5 mM. Store aliquots at -20 °C
24. Brain-derived neurotrophic factor (BDNF) (Cedarlane Labs, catalog number: CLY100-01-100UG); reconstitute in 1 mL of dH2O + 0.1% BSA to achieve a 100 mg/mL solution, and store aliquots at -20 °C
25. Glial cell line-derived neurotrophic factor (GDNF) (Cedarlane Labs, catalog number: CLY100-02-100UG); reconstitute in 1 mL of dH2O + 0.1% BSA to achieve a 100 mg/mL solution, and store aliquots at -20 °C
26. Essential 8TM Medium (E8) (ThermoFisher Scientific, catalog number: A1517001); see Recipe 5 for details
27. Human recombinant bone morphogenic protein 4 (BMP-4) (STEMCELL Technologies, catalog number: 78211); reconstitute in 40 μL of dH2O to achieve a 500 μg/mL solution and store aliquots at -20 °C
28. Recombinant human vascular endothelial growth factor 121 (VEGF-121) (BioLegend, catalog number: 583202); sold as 200 μg/mL, aliquot and store at -20 °C
29. Gibco stem cell factor (C Kit Ligand) recombinant human protein (SCF) (ThermoFisher Scientific, catalog number: PHC2115); reconstitute in 50 μL of dH2O BSA to achieve a 200 μg/mL solution, and store aliquots at -20 °C
30. X-VIVOTM 15 serum-free hematopoietic cell medium, sterile, pH 6.9–7.2, with L-Glutamine, gentamicin, and phenol red, liquid (X-VIVO 15) (Lonza, catalog number: 04-418Q); store at 4 °C away from light
31. Human macrophage colony-stimulating factor recombinant protein (M-CSF) (ThermoFisher Scientific, catalog number: PHC9501); reconstitute in 100 μL of dH2O to achieve a 1 mg/mL solution, and store aliquots at -20 °C
32. Human recombinant IL-3 (IL-3) (STEMCELL Technologies, catalog number: 78040); reconstitute in 400 μL of dH2O to achieve a 250 μg/mL solution, and store aliquots at -20 °C
33. Advanced DMEM/F12 (ThermoFisher Scientific, catalog number: 12634010); store at 4 °C
34. Human recombinant granulocyte-macrophage colony-stimulating factor (E. coli-expressed) (GM-CSF) (STEMCELL Technologies, catalog number: 78015.1); reconstitute in 200 μL of dH2O to achieve a 100 μg/mL solution, and store aliquots at -20 °C
35. Recombinant human IL-34 (carrier-free) 100 μg (IL-34) (BioLegend, catalog number: 577906); sold at varying concentrations ~1 mg/mL. If above this concentration, adjust by adding dH2O to achieve 1 mg/mL and follow Recipe 7. If below, adjust Recipe 7 as needed to compensate
36. Recombinant human TGF-β1 (HEK293 derived) (TGF-β1) (PeproTech, catalog number: 100-21-250UG); reconstitute in 500 μL of dH2O to achieve a 500 μg/mL solution, and store aliquots at -20 °C
37. TrypLETM Express enzyme (1×), no phenol red (TrypLE) (ThermoFisher Scientific, catalog number: 12604039); store at RT
38. Versene solution (ThermoFisher Scientific, catalog number: 15040066); store at 4 °C
Solutions
1. Basal neural maintenance medium (BNMM) (see Recipes)
2. Neurosphere expansion medium (NSE) (see Recipes)
3. Neurosphere maturation medium (NSM) (see Recipes)
4. Neurosphere differentiation medium (NSD) (see Recipes)
5. Embryoid body medium (EBM) (see Recipes)
6. Hematopoietic differentiation medium (HDM) (see Recipes)
7. Microglial differentiation medium (MDM) (see Recipes)
Recipes
Note: Many of the recipes listed below require additional supplements added fresh before use. If this is the case, supplements will be listed alongside the recipe name within the Procedure (e.g., BNMM + 10 μM Y-27632 means BNMM with Y-27632 added immediately before changing media to reach a final concentration of 10 μM).
1. Basal neural maintenance medium (BNMM)
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| DMEM/F12 | n/a (base) | 239.8 mL |
| Neurobasal | n/a (base) | 239.8 mL |
| B-27 | 1% | 5 mL |
| N-2 | 0.5% | 2.5 mL |
| Pen/Strep | 1% (100 IU) | 5 mL |
| GlutaMAX | 0.5% | 2.5 mL |
| MEM-NEAA | 0.5% | 2.5 mL |
| ITS-A | 0.5% | 2.5 mL |
| 2-mercaptoethanol | 0.08% | 400 μL |
| Total | n/a | 500 mL |
a. Thaw frozen supplements at room temperature (RT).
b. Filter-sterilize using a 500 mL vacuum filter bottle.
c. Keep media at 4 °C and use within 1 month of being mixed.
d. If a smaller volume is desired, media recipe can be reduced by 10× and filter-sterilized using a Steriflip® vacuum tube top filter.
2. Neurosphere expansion medium (NSE)
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| DMEM/F12 | n/a (base) | 236.25 mL |
| Neurobasal | n/a (base) | 236.25 mL |
| B-27 | 2% | 10 mL |
| N-2 | 1% | 5 mL |
| Pen/Strep | 1% (100 IU) | 5 mL |
| GlutaMAX | 0.5% | 2.5 mL |
| MEM-NEAA | 0.5% | 2.5 mL |
| ITS-A | 0.5% | 2.5 mL |
| Total | n/a | 500 mL |
a. Thaw frozen supplements at RT. Filter-sterilize using a 500 mL vacuum filter bottle.
b. Keep media at 4 °C and use within 1 month of being mixed.
c. If a smaller volume is desired, the recipe can be reduced by 10× and filter-sterilized using a Steriflip® vacuum tube top filter.
3. Neurosphere maturation medium (NSM)
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| BrainPhys | n/a (base) | 480 mL |
| SM1 | 2% | 10 mL |
| N2-A | 1% | 5 mL |
| Pen/Strep | 1% (100 IU) | 5 mL |
| Total | n/a | 500 mL |
a. Thaw frozen supplements at RT. Filter-sterilize using a 500 mL vacuum filter bottle.
b. Keep media at 4 °C and use within 1 month of being mixed.
c. If a smaller volume is desired, the recipe can be reduced by 10× and filter-sterilized using a Steriflip® vacuum tube top filter.
4. Neurosphere differentiation medium (NSD)
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| BrainPhys | n/a (base) | 484 mL |
| SM1 | 2% | 10 mL |
| N2-A | 1% | 5 mL |
| Pen/Strep | 1% (100 IU) | 5 mL |
| cAMP (50 mg/mL) | 500 μg/mL | 5 mL |
| Ascorbic acid (100 μM) | 200 nM | 1 mL |
| Total | n/a | 500 mL |
a. Thaw frozen supplements at RT. Filter-sterilize using a 500 mL vacuum filter bottle.
b. Keep media at 4 °C and use within 1 month of being mixed.
c. If a smaller volume is desired, the recipe can be reduced by 10× and filter-sterilized using a Steriflip® vacuum tube top filter.
d. Note that NSD only differs from NSM in cAMP and ascorbic acid, so an alternative strategy is to add these supplements to already mixed NSM at the listed ratios.
5. Embryoid body medium (EBM)
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| E8 base media | n/a (base) | 9.79 mL |
| E8 Supplement | 2% | 0.2 mL |
| BMP4 (500 μg/mL) | 50 ng/mL | 1 μL |
| SCF (200 μg/mL) | 20 ng/mL | 1 μL |
| VEGF-121 (200 μg/mL) | 50 ng/mL | 2.5 μL |
| Y-27632 (10 mM) | 10 μM | 10 μL |
| Total | 10 mL |
Critical: E8 medium is provided as a base medium (500 mL) and supplement (10 mL). We have found that it is necessary to immediately freeze both the base medium in 50 mL aliquots and the supplement in 1 mL aliquots at -20 °C. Thaw all components at RT and use within 1 week of mixing.
6. Hematopoietic differentiation medium (HDM)
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| X-VIVO 15 | n/a (base) | 48.94 mL |
| GlutaMAX | 1% | 0.5 mL |
| Pen/Strep | 1% (100 IU) | 0.5 mL |
| 2-mercaptoethanol | 0.1% | 50 μL |
| M-CSF (1 mg/mL) | 100 ng/mL | 5 μL |
| IL-3 (250 μg/mL) | 25 ng/mL | 5 μL |
| Total | 50 mL |
Thaw frozen supplements at RT. Keep media at 4 °C and use within 1 month of being mixed.
7. Microglial differentiation medium (MDM)
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Advanced DMEM/F12 | n/a (base) | 48.98 mL |
| GlutaMAX | 1% | 0.5 mL |
| Pen/Strep | 1% | 0.5 mL |
| GM-CSF (100 μg/mL) | 10 ng/mL | 5 μL |
| IL-34 (1 mg/mL) | 100 ng/mL | 5 μL |
| TGFβ1 (500 μg/mL) | 50 ng/mL | 5 μL |
| M-CSF (1 mg/mL) | 25 ng/mL | 1.25 μL |
| Total | 50 mL |
Thaw frozen supplements at RT. Keep media at 4 °C and use within 2 weeks of being mixed.
Laboratory supplies
1. Corning® Isotip® filtered pipette tips, 0.1–2 µL (Millipore Sigma, catalog number: CLS4801-960EA)
2. Axygen 20 μL maximum recovery universal fit filter tips racked, clear, sterile, rack pack (VWR, catalog number: 22234-008)
3. 200 μL filter tips retention, sterile rack, 96 tips/rack, 10 racks/pack (ESBE Scientific, catalog number: ESB-VAR200200PT)
4. 1,000 μL filter tips retention, sterile rack, 96 tips/rack, 10 racks/pack (ESBE Scientific, catalog number: ESB-VAR201000PT)
5. Optifit P1000 wide-bore tips, Sartorius (VWR, catalog number: CA15000-468)
6. Thermo Scientific ClipTipTM 1,250 µL filtered pipette tips (ThermoFisher Scientific, catalog number: 94420813)
7. NuncTM serological pipettes, 5 mL (Tapered tip) (Thermo Scientific, catalog number: 170355N)
8. NuncTM serological pipettes, 10 mL (Tapered tip) (Thermo Scientific, catalog number: 170356N)
9. Thermo ScientificTM NuncTM serological pipettes, 25 mL (Thermo Fisher Scientific, catalog number: 170357N)
10. Falcon® 6-well polystyrene microplate (ThermoFisher Scientific, catalog number: 08-772-33)
11. Falcon® 15 mL conical bottom centrifuge tube (VWR, catalog number: CA21008-918)
12. Falcon® 50 mL conical bottom centrifuge tube (VWR, catalog number: CA21008-951)
13. Vacuum filtration systems 500 mL (VWR, catalog number: 10040-436)
14. Steriflip-GV, 0.22 μm, PVDF, radio-sterilized (EMD Millipore, catalog number: SE1M179M6)
15. Mr. FrostyTM freezing container (ThermoFisher Scientific, catalog number: 5100-0001)
16. AggreWellTM 800 Microwell 24-well culture plates (STEMCELL Technologies, catalog number: 34815)
17. Large 37 μm reversible strainer (STEMCELL Technologies, catalog number: 27250)
18. NuncTM EasYFlaskTM 75 cm2 polystyrene tissue culture treated flasks, sterile, filter cap, angled neck (ThermoFisher Scientific, catalog number: 156499)
19. Cellvis 6-well glass bottom plate with high performance #1.5 cover glass, 20 pack (ThermoFisher Scientific, catalog number: NC0452316)
20. Vwr® tissue culture dish, non-treated, sterilized, non-pyrogenic, diameter 9.0 cm, growth area 55 cm2 (VWR, catalog number: 10861-592)
21. VWR® disposable pipetting reservoirs (sterile) (VWR, catalog number: 89108-002)
22. Falcon® cell scrapers, sterile (Corning, catalog number: CA15621-005)
Equipment
Note: Only specific, more specialized cell culture equipment that may not be found in every cell culture lab space is listed below. Basic cell culture equipment (BSC hood, incubator, etc.) is required but omitted from the following list.
1. Incubator
2. Tissue culture hood
3. Light microscope
4. Dissection microscope (ZEISS, model: SteREO Discovery.V8 or RWD Life Science, DOM 1001)
5. Gilson Pipetman L 4-Pack Starter Kit, P2L, P20L, P200L, P1000L (Mandel, catalog number: GF-F167370)
6. Pipet Aid® Portable pipetting device, 110 V, Drummond Scientific 4-000-100, 1/EA (ThermoFisher Scientific, catalog number: 13-681-19)
7. E1-ClipTip; Bluetooth; electronic adjustable tip spacing multichannel equalizer pipettes (ThermoFisher Scientific, catalog number: 4672090BT)
8. Celltron orbital shaker system (Infors HT)
Procedure
文章信息
稿件历史记录
提交日期: Jul 31, 2025
接收日期: Sep 25, 2025
在线发布日期: Oct 14, 2025
出版日期: Nov 5, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/).
如何引用
Wendt, S., Lee, C., Cai, W., Lin, A. J., Huang, J., Poon, V., Xiang, X., Hong, W., MacVicar, B. A. and Nygaard, H. B. (2025). Generation of 3D Human iPSC-Derived Multi-Cell Type Neurospheres for Studying Neuron, Astrocyte, and Microglia Crosstalk. Bio-protocol 15(21): e5493. DOI: 10.21769/BioProtoc.5493.
分类
神经科学 > 细胞机理 > 小神经胶质
干细胞 > 成体干细胞 > 神经干细胞
细胞生物学 > 细胞工程 > 组织工程
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link