- EN - English
- CN - 中文
A Reliable In Planta Inoculation and Antifungal Screening Protocol for Rhizoctonia solani-Induced Sheath Blight in Rice
水稻纹枯病的体内接种与抗真菌筛选可靠方法
发布: 2025年11月05日第15卷第21期 DOI: 10.21769/BioProtoc.5491 浏览次数: 1562
评审: Shweta PanchalAnonymous reviewer(s)

相关实验方案
微生物提取物对卵菌辣椒疫霉菌和猝倒病疫霉的体外筛选
Mónica Trigal Martínez [...] María Ángeles Vinuesa Navarro
2025年09月20日 1261 阅读
Abstract
Sheath blight, caused by Rhizoctonia solani, is a major fungal disease of rice that leads to significant yield losses globally. Conventional inoculation methods often fail to achieve consistent and uniform infection, limiting their applicability in antifungal screening studies. This protocol describes a reliable in planta inoculation method for R. solani using mature sclerotia placed at the internodal region of tillering-stage rice seedlings. The procedure includes step-by-step instructions for seed germination, seedling preparation, pathogen culture, artificial inoculation, and post-infection application of antifungal treatments, including botanical compounds such as Ocimum gratissimum essential oil and thymol. Lesion development is monitored and quantified over time, and data are analyzed statistically to evaluate treatment efficacy. The protocol is optimized for reproducibility, scalability, and compatibility with sustainable disease management approaches. It provides a robust platform for evaluating antifungal agents in a biologically relevant and controlled environment.
Key features
• Establishes a reliable in planta inoculation method for R. solani in rice, overcoming the common challenge of achieving consistent disease development.
• Enables post-inoculation screening of botanicals for antifungal efficacy under realistic plant–pathogen interaction conditions.
• Integrates sustainable research practices by detailing botanical extraction and their in planta assessment against R. solani infection.
Keywords: Rice (水稻)Graphical overview
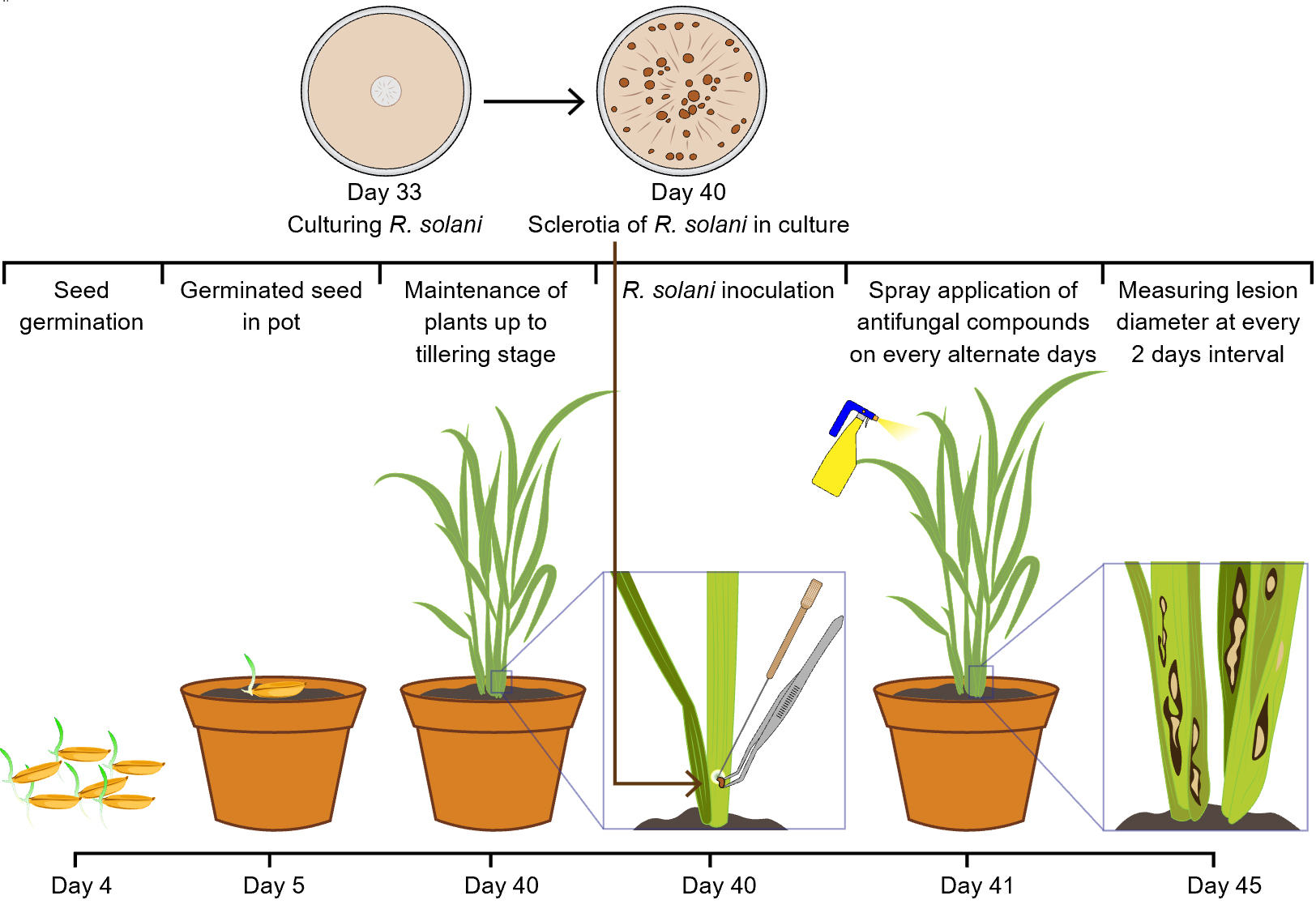
Background
Rhizoctonia solani Kühn, an economically significant necrotrophic pathogen, is the causal agent of sheath blight in rice (Oryza sativa), one of the most devastating diseases affecting rice cultivation globally [1]. The pathogen infects the leaf sheath and other aerial parts of the plant, especially during the vegetative phase, leading to reduced photosynthetic area, premature senescence, and ultimately substantial yield loss [2]. Despite extensive breeding efforts, no rice cultivar exhibits complete resistance to R. solani, making effective disease management reliant on agronomic practices and chemical control [3]. The in-depth study of the rice–R. solani pathosystem, including host response, virulence mechanisms, and the evaluation of biocontrol or botanical treatments, requires a dependable in planta inoculation method that closely mimics field conditions. However, this has remained a technical challenge due to the pathogen’s facultative saprophytic nature and sensitivity to environmental cues like temperature, humidity, and plant developmental stage.
Various methods have been previously employed to inoculate R. solani under experimental conditions, each with notable limitations. Mycelial plug placement on the leaf sheath is a common method that provides localized infection but lacks uniformity and is labor-intensive for large-scale screening [4]. Soil inoculation using pathogen-colonized rice straw, barley grains, or toothpicks can introduce the pathogen to the plant base but often results in variable infection levels and non-uniform disease progression [5–7]. Spraying of conidial or mycelial suspensions is not feasible in this case, as R. solani does not produce conidia under typical conditions, and foliar application of mycelial fragments tends to dry out quickly, hampering effective colonization [8]. The detached leaf assay is another frequently used approach for preliminary antifungal screenings. While it is rapid and suitable for high-throughput screening, it fails to capture the systemic plant responses and physiological interactions that occur in whole-plant systems, thereby limiting its translational value to field scenarios.
This protocol addresses these issues by providing a step-by-step methodology for consistent in planta inoculation of R. solani in rice at the tillering stage, an important developmental phase when the plant is most susceptible to sheath blight infection. The method ensures robust symptom development by using freshly prepared pathogen cultures placed under controlled humidity and temperature conditions to simulate conducive environments for infection. It is optimized for reproducibility and can be employed for both small- and medium-scale experimental setups. A unique strength of this protocol is its integration with post-inoculation antifungal evaluation using botanical extracts. With increasing concerns over fungicide resistance and environmental toxicity associated with synthetic chemicals, plant-derived antifungal compounds are gaining prominence as eco-friendly alternatives. While several studies have reported in vitro antifungal activity of essential oils and plant extracts against R. solani, their performance in planta remains underexplored. This protocol provides a reliable and biologically relevant framework for assessing the efficacy of such botanicals following real-time infection, thus enhancing the translational significance of in vitro findings.
In addition to antifungal screening, this inoculation method is adaptable for various downstream applications, including microscopy for fungal colonization, gene expression analysis of defense pathways, histochemical staining, and metabolic profiling during pathogen challenge. It thus supports a wide array of studies aimed at understanding host–pathogen dynamics, resistance mechanisms, and the development of sustainable disease control strategies.
Materials and reagents
Biological materials
1. Virulent strain of Rhizoctonia solani OR230677.1 (origin: ARRI, Titabor, Assam, India)
2. Any susceptible rice (Oryza sativa) cultivar such as Swarna (MTU 7029), Samba Mahsuri (BPT 5204), or Pusa Basmati 1 (PB-1)
Reagents
1. Double-distilled water
2. Ethanol 99.5%, analytical grade (Changshu Hongsheng Fine Chemical Co., Ltd)
3. Ocimum gratissimum essential oil (extracted by hydrodistillation using Clevenger type apparatus [9])
4. Thymol (HiMedia, CAS number: GRM7581-100G)
5. Tween 80 (SRL, CAS number: 9005-65-6)
6. Propiconazole (Syngenta, Tilt, CAS number: 60207-90-1)
7. Potato dextrose agar medium (PDA) (HiMedia, CAS number: GM096-500G)
Solutions
1. Potato dextrose agar medium (PDA) (see Recipes)
2. Ethanol (70%) (see Recipes)
3. Tween 80 (0.25%) solution (see Recipes)
4. Ocimum gratissimum essential oil in 0.25% Tween 80 (1,000 ppm) (see Recipes)
5. Thymol in 0.25% Tween 80 (1,000 ppm) (see Recipes)
6. Propiconazole in 0.25% Tween 80 (1,000 ppm) (see Recipes)
Recipes
1. PDA medium
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| PDA (commercial powder) | 39 g/L | 39 g |
| Double-distilled water | - | Up to 1,000 mL |
| Total | n/a | 1,000 mL |
a. Weigh 39 g of commercially available PDA powder and transfer it to a sterile 1 L borosilicate flask.
b. Add double-distilled water to bring the total volume to 1,000 mL.
c. Stir thoroughly until the powder is completely dissolved. Heat gently if necessary.
d. Adjust the pH to 5.6 ± 0.2 using sterile 1 N NaOH or HCl.
e. Dispense the medium into autoclavable containers and sterilize at 121 °C, 15 psi for 15 min.
f. After sterilization, allow the medium to cool to approximately 50 °C by placing it on the laminar airflow bench with occasional gentle stirring; then, pour into sterile Petri dishes.
g. Store the plates inverted at 4 °C in the dark and use within 2–3 weeks.
2. Ethanol (70%)
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Absolute ethanol (≥99.5%) | 70% (v/v) | 700 mL |
| Double-distilled water | 30% (v/v) | 300 mL |
| Total | n/a | 1,000 mL |
a. To prepare 1,000 mL of 70% ethanol, measure 700 mL of absolute ethanol (≥99.5%) using a measuring cylinder and transfer it to a clean, sterile glass beaker.
b. Add 300 mL of double-distilled water to reach a final volume of 1,000 mL.
c. Mix thoroughly by swirling or gentle stirring.
d. Store the solution in a tightly sealed container at room temperature.
e. Label the container with preparation date and concentration.
f. Use freshly prepared solution or store for up to 6 months.
3. Tween 80 (0.25%) solution
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Tween 80 | 0.25% (v/v) | 2.5 mL |
| Double-distilled water | - | Up to 1,000 mL |
| Total | n/a | 1,000 mL |
a. To prepare 1,000 mL of 0.25% Tween 80 solution, measure 2.5 mL of Tween 80 using a sterile pipette and add it to approximately 900 mL of double-distilled water in a clean, sterile beaker.
b. Mix thoroughly using a magnetic stirrer or by gentle inversion until the solution is homogeneous.
c. Adjust the final volume to 1,000 mL with additional double-distilled water.
d. Sterilize the solution by autoclaving at 121 °C, 15 psi for 15 min.
e. Store at room temperature or 4 °C, protected from light.
4. Ocimum gratissimum essential oil in 0.25% Tween 80 (1,000 ppm)
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Ocimum gratissimum essential oil | 1,000 ppm (v/v) | 1.0 mL |
| 0.25% Tween 80 solution (see Recipe 3) | - | 999 mL |
| Total | n/a | 1,000 mL |
a. To prepare 1,000 mL of Ocimum gratissimum essential oil solution at 1,000 ppm, first prepare 0.25% Tween 80 solution as described in Recipe 3.
b. Then, add 1.0 mL of Ocimum gratissimum essential oil to 999 mL of 0.25% Tween 80 solution.
c. Mix thoroughly using a magnetic stirrer or by vortexing until the oil is uniformly emulsified.
d. Use freshly prepared or store at 4 °C in a sealed amber bottle to protect from light.
5. Thymol in 0.25% Tween 80 (1,000 ppm)
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Thymol | 1,000 ppm (w/v) | 1.0 g |
| 0.25% Tween 80 solution (see Recipe 3) | - | 999 mL |
| Total | n/a | 1,000 mL |
a. To prepare 1,000 mL of thymol solution at 1,000 ppm, weigh 1.0 g of thymol and dissolve it in 999 mL of 0.25% Tween 80 solution, prepared as described in Recipe 3.
b. Mix thoroughly by stirring or vortexing until completely dissolved.
c. Store in a sealed container at 4 °C and protect from light.
6. Propiconazole in 0.25% Tween 80 (1,000 ppm)
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Propiconazole (a.i.) | 1,000 ppm (v/v) | 1.0 mL |
| 0.25% Tween 80 solution (see Recipe 3) | - | 999 mL |
| Total | n/a | 1,000 mL |
a. To prepare 1,000 mL of propiconazole solution at 1,000 ppm, pipette 1.0 mL of commercial-grade propiconazole (active ingredient, a.i.) and mix into 999 mL of 0.25% Tween 80 solution prepared as described in Recipe 3.
b. Mix thoroughly using a magnetic stirrer or vortex to obtain a uniform solution.
c. Store at 4 °C in a tightly sealed, light-protected container and use within 2 weeks.
Laboratory supplies
1. Plastic pots (15 cm diameter)
2. Sterile Petri dishes (Tarson, catalog number: 100 mm PP Autoclavable Petri Dish 46203)
3. Sterile muslin cloth (Euqinu 1 × 1 Meter Cotton Muslin Cloth, Ultra Fine Fabric)
4. Sterile forceps (Vandersluys normal tip forceps)
5. Sterile scalpel or inoculation loop (Tarsons, catalog number: 920051)
6. Sterile needle (Thomas Scientific, needle with nichrome wire)
7. Hand sprayers (one per treatment) (1.5 L Garden Agriculture Spray Pump Atomizer Compression Pressure Sprayer)
8. pH indicator strips (Parshv, model number: P.H. [Universal] Indicator Paper)
9. Measuring cylinders (100 mL) (Borosil, catalog number: 3022016)
10. Transparent ruler or digital caliper (UniMarket, model number: UniMarketCH2-9MDPD26T)
11. Borosilicate glass flasks (1 L) (Borosil, catalog number: 5360029)
12. Sterile beakers (1,000 mL) (Borosil, model number: ZGB-08)
13. Micropipettes (10 µL, 20 µL, 200 µL, 1,000 µL) (Tarsons)
14. Corresponding sterile pipette tips (10 µL, 20 µL, 200 µL, 1,000 µL) (Tarsons)
15. Disposable gloves
16. Laboratory masks (AMPri, catalog number: 081307-L)
17. Lab coat
18. Waste disposal bags
Equipment
1. Greenhouse or plant growth chamber with environmental control (temperature, humidity, light cycle) (iGene, model number: IG-150GC)
2. Incubator (capable of maintaining 33 °C) (GENEXIS, model: GLA-109-2)
3. Autoclave (for sterilization at 121 °C, 15 psi) (EQUITRON, model: Portable PAD)
4. Magnetic stirrer with stir bars (REMI, model: 2-MLH)
5. pH meter (Mettler-Toledo GmbH, model: FP20)
6. Analytical balance (SCALETEC, model: SAB-203L)
7. Vortex mixer (Ambinova, model: AMBI-VM-01)
8. Laminar airflow cabinet (for aseptic work) (Ambinova, model: AMBI-LAF-3999HE)
9. Refrigerator (4 °C, for media and inoculum storage) (SAMSUNG, model: RT39B551ES8/HL)
10. Digital camera (for lesion imaging and documentation)
Software and datasets
1. RStudio (Posit, version 2025.05.1+513)
Procedure
文章信息
稿件历史记录
提交日期: Jun 28, 2025
接收日期: Sep 16, 2025
在线发布日期: Oct 14, 2025
出版日期: Nov 5, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Yasin, A., Kashyap, A., Dowarah, B., Dey, J., Bharali, S., Nath, B. C., Bhattacharyya, P. N., Sharma, S., Barua, P. and Nath, P. D. (2025). A Reliable In Planta Inoculation and Antifungal Screening Protocol for Rhizoctonia solani-Induced Sheath Blight in Rice. Bio-protocol 15(21): e5491. DOI: 10.21769/BioProtoc.5491.
分类
植物科学 > 植物免疫 > 宿主-细菌相互作用
微生物学 > 微生物-宿主相互作用 > 真菌
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link