- EN - English
- CN - 中文
Enhancement of RNA Imaging Platforms by the Use of Peptide Nucleic Acid-Based Linkers
发布: 2026年05月05日第16卷第9期 DOI: 10.21769/BioProtoc.5453 浏览次数: 1438
评审: Marion HoggAnonymous reviewer(s)
Abstract
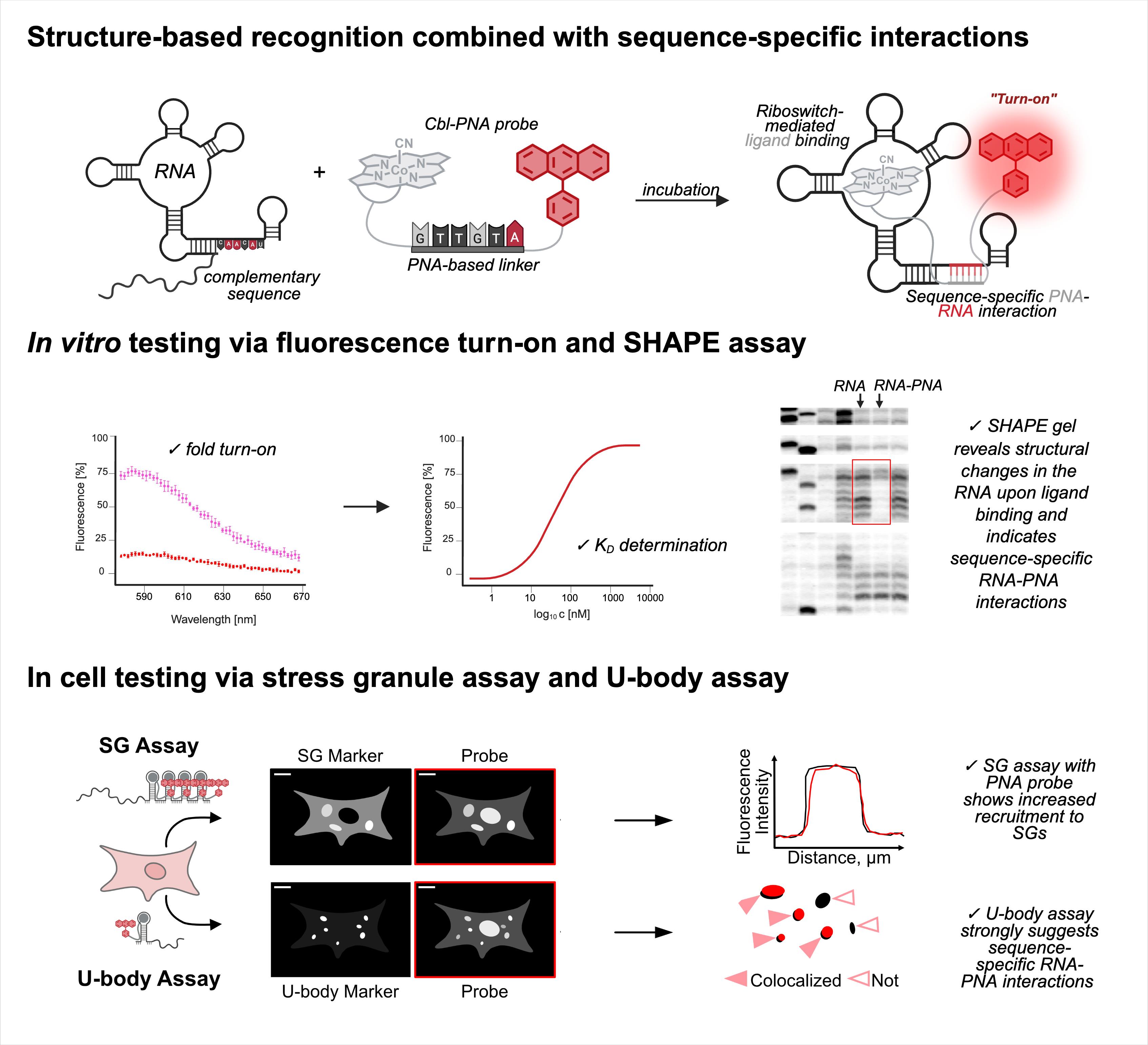
RNA imaging techniques enable researchers to monitor RNA localization, dynamics, and regulation in live or fixed cells. While the MS2-MCP system—comprising the MS2 RNA hairpin and its binding partner, the MS2 coat protein (MCP)—remains the most widely used approach, it relies on a tag containing multiple fluorescent proteins and has several limitations, including the potential to perturb RNA function due to the tag’s large mass. Alternative methods using small-molecule binding aptamers have been developed to address these challenges. This protocol describes the synthesis and characterization of RNA-targeting probes incorporating a peptide nucleic acid (PNA)-based linker within the cobalamin (Cbl)-based probe of the Riboglow platform. Characterization in vitro involves a fluorescence turn-on assay to determine binding affinity (KD) and selective 2′-hydroxyl acylation analyzed by primer extension (SHAPE) footprinting analysis to assess RNA-probe interactions at a single nucleotide resolution. To show the advancement of PNA probes in live cells, we present a detailed approach to perform both stress granule (SG) and U-body assays. By combining sequence-specific hybridization with structure-based recognition, our approach enhances probe affinity and specificity while minimizing disruption to native RNA behavior, offering a robust alternative to protein-based RNA imaging systems.
Key features
• Contains four parts, describing I) cobalamin-PNA probe synthesis, II) fluorescence turn-on assay, III) SHAPE assay, and IV) stress granule (SG) and U-body assays.
• Enables high-specificity RNA imaging through avidity, using cobalamin-based probes that incorporate peptide nucleic acid (PNA) linkers for sequence-specific hybridization with the RNA tag.
• Increases the dynamic range for the stress granule (SG) assay used in the field to evaluate a fluorescent RNA tag’s ability to visualize RNA localization.
• Provides insights on how to adapt the described procedures to other RNA-small molecule pairs.
Keywords: CobalaminGraphical overview
Background
The ability to visualize macromolecules in live cells has significantly advanced our understanding of cellular processes. While protein imaging has benefited from a wide range of genetically encoded fluorescent tags, live-cell RNA imaging remains more technically challenging and less developed. The most widely used tool for RNA visualization, the MS2-MCP system [1,2], relies on the binding of fluorescent proteins to engineered stem loops appended to the RNA of interest. Although powerful, this system suffers from several limitations, including potential perturbation of RNA function, limited signal-to-noise ratio, and challenges with multiplexing [3–5].
To address these issues, alternative RNA imaging tools have been developed that utilize RNA aptamers capable of binding small-molecule fluorophores or fluorophore-quencher conjugates. Among these, systems such as Peppers [6], RhoBAST [7], Spinach [8], Mango [9], and Riboglow [10] offer genetically encodable tags that fluoresce upon binding to their cognate ligands. Riboglow, in particular, uses a well-folding bacterial class-II cobalamin (Cbl)-binding riboswitch [11] as the RNA aptamer and a Cbl-fluorophore conjugate as the probe. In the Riboglow system, RNAs of interest are tagged with 1–4 copies of a 102-nt (33 kDa) cobalamin riboswitch that binds ~1:1 with 4.1 kDa Cbl-fluorophore probe conjugates. In contrast, the MS2-MCP system typically uses twelve or more 23-nt (7.4 kDa) MS2 stem loops that bind ~1:2 with ~40-kDa MCP-fluorescent protein conjugates. Thus, the Riboglow system introduces less total mass to an RNA of interest, potentially reducing interference with its native function. Further, upon aptamer binding, the fluorophore is spatially separated from the conjugated quencher (here, cobalamin), leading to fluorescence activation. While this system improves signal responsiveness and flexibility, its modular design has historically relied on polyethylene glycol (PEG) linkers, which are chemically inert and primarily serve as spacers.
Our recent work addresses a limitation in existing RNA imaging probe design by replacing the PEG linker in the Riboglow platform with a short peptide nucleic acid (PNA) sequence capable of base pairing with the RNA target [12]. PNAs are synthetic DNA mimics with neutral backbones, offering high binding affinity and resistance to nucleases [13]. By incorporating a six-nucleotide PNA linker complementary to a single-stranded region of the RNA aptamer (outside the Cbl-binding pocket), we introduce an additional recognition mechanism that enhances probe-RNA affinity through avidity. This dual-binding design improves affinity and enables detection of truncated aptamer variants with significantly higher sensitivity. Compared to existing methodologies, this approach offers several advantages: a) increased probe affinity through sequence-specific hybridization, b) enhanced modularity and design flexibility via tunable PNA sequences, c) improved performance in live-cell imaging, particularly in detecting RNA localized to membrane-less organelles such as stress granules (SGs), and d) compatibility with shorter or mutated RNA aptamers that would otherwise compromise probe binding [12].
The pipeline described here enables a thorough and detailed examination of the efficiency of an RNA imaging platform that combines sequence-specific hybridization with structure-based recognition. We divided the protocol into four parts (I–IV), which can be used independently or as sequential parts of the overall workflow. To enhance readability and usability, each section includes its own set of required materials, procedure description, data analysis, notes, and troubleshooting information (if applicable). We describe in detail the Cbl-PNA-based probe synthesis (Part I), in vitro testing against relevant RNA via fluorescence turn-on assay (Part II), and SHAPE gel experiments (Part III) [12]. Cell-based studies (Part IV) focus on an SG assay and a U-body assay to evaluate the ability of the Cbl-PNA probes to visualize RNA localization and dynamics [12]. The SG assay protocol described here improves upon the assay used in the field [6,10,14–20] by increasing the dynamic range of the resultant data [12]. The U-body assay protocol largely follows similar protocols already present in the literature [10,17], but we demonstrate that this assay can be used to quantify the effects of each binding mode in live cells [12].
The assays described here are not limited to Cbl probes and native cobalamin riboswitches (here, env8); they can be adapted to any RNA-probe pair. It should be noted that the in vitro fluorescence turn-on assay to determine the dissociation constant requires that the probe exhibit an increase in fluorescence upon binding to the RNA.
Part I: Cobalamin-PNA probe synthesis
Materials and reagents
Reagents
1. 1-(2-aminoethyl)-1H-pyrrole-2,5-dione, TFA salt (Ambeed, catalog number: A275035)
2. 1,1-Carbonyldi-(1,2,4-triazole) (CDT) (Chem-Impex, catalog number: 14114)
3. 6-(tritylthio)hexanoic acid (BroadPharm, catalog number: BP-25464)
4. ATTO590 alkyne (ATTO-TEC, catalog number: AD590)
5. Copper(I) iodide (CuI) (Sigma-Aldrich, catalog number: 792063)
6. Cyanocobalamin (Sigma-Aldrich, catalog number: V2876)
7. Fmoc-Lys(N3)-OH (Chem-Impex, catalog number: 29756)
8. N,N-Diisopropylethylamine (DIPEA) (Acros Organics, catalog number: 115220250)
9. PNA monomers: Fmoc-A(Bhoc)-OH (PNA Bio, catalog number: FMA-1001), Fmoc-C(Bhoc)-OH (PNA Bio, catalog number: FMC-1001), Fmoc-G(Bhoc)-OH (PNA Bio, catalog number: FMG-1001), and Fmoc-T-OH (PNA Bio, catalog number: FMT-1001)
10. Reagents for coupling reactions on the resin: 1-hydroxy-7-azabenzotriazole (HOAt) (aablocks, catalog number: AA0032PK), 2-(7-aza-1H-benzotriazole-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate (HATU) (Ambeed, catalog number: A633512), 2,4,6-collidine (Ambeed, catalog number: A208876), 2,6-lutidine (TCI, catalog number: L0067), 4-dimethylaminopyridine (DMAP) (Ambeed, catalog number: A538667), and 4-methylmorpholine (NMM) (TCI, catalog number: M0370)
11. Reagents for Fmoc deprotection and cleavage solution: m-cresol (Sigma-Aldrich, catalog number: C85727), piperidine (Chem-Impex, catalog number: 02351), trifluoroacetic acid (Sigma-Aldrich, catalog number: T6508), triisopropylsilane (TIS) (Ambeed, catalog number: A187865)
12. Rink amide resin, 0.3–0.6 meq/g, 100–200 mesh (Chem-Impex, catalog number: 12662)
13. Solvents: acetonitrile (MeCN) (HPLC grade) (Fisher Chemical, catalog number: A998-4), dichloromethane (DCM) (Sigma-Aldrich, catalog number: 270997), diethyl ether (Et2O) (VWR, catalog number: BDH1121-1LPC), dimethyl formamide (DMF) (Acros Organics, catalog number: 348430010), dimethyl sulfoxide (DMSO) (Sigma-Aldrich, catalog number: 276855), ethyl acetate (AcOEt) (Fisher Chemical, catalog number: E145-4), H2O (HPLC grade) (Macron, catalog number: 6795-10), methanol (MeOH) (VWR, catalog number: BDH1135-4LP), N-Methyl-2-pyrrolidone (NMP) (Thermo Scientific, catalog number: 364381000), potassium phosphate monobasic buffer (pH = 7) (Fisher Chemical, catalog number: SB107-500)
14. Tris[(1-benzyl-1H-1,2,3-triazol-4 yl)methyl]amine (TBTA) (Sigma-Aldrich, catalog number: 678937)
Solutions
1. Cleavage solution (see Recipes)
2. Coupling solution (see Recipes)
3. Fmoc deprotection solution (see Recipes)
Recipes
1. Cleavage solution
Caution: TFA, TIS, and m-cresol are hazardous reagents that are corrosive, toxic, or flammable; handle them in a fume hood with appropriate personal protective equipment (PPE) and avoid inhalation or skin contact. Use a 1 oz glass bottle with a cap to store the cleavage solution.
| Reagent | Final concentration | Volume of stock |
|---|---|---|
| TFA | 95% | 5.7 mL |
| TIS | 2.5% | 150 μL |
| m-cresol | 2.5% | 150 μL |
| Total | n/a | 6 mL |
2. Coupling solution
The coupling solution is prepared using dry solvents. Follow standard dry-lab techniques when handling and transferring solvents. Store the coupling solution in a capped 2 oz glass bottle and purge with inert gas between coupling steps to preserve the solvents’ integrity. Handle solvents in a fume hood with appropriate PPE.
| Reagent | Final concentration | Volume of stock |
|---|---|---|
| DMF | 50% | 10 mL |
| NMP | 50% | 10 mL |
| Total | n/a | 20 mL |
3. Fmoc deprotection solution
The Fmoc deprotection solution is prepared using dry DMF. Follow standard dry-lab techniques when handling and transferring solvents. Store the solution in a capped 6 oz glass bottle and purge with inert gas between deprotection steps to preserve the solvents’ integrity.
Caution: Piperidine is toxic, volatile, and corrosive; handle it in a fume hood with appropriate PPE.
| Reagent | Final concentration | Volume of stock |
|---|---|---|
| DMF | 80% | 64 mL |
| Piperidine | 20% | 16 mL |
| Total | n/a | 80 mL |
Laboratory supplies
1. C18-reversed phase silica gel, LiChroprep RP-18 25-40 μm (Millipore Sigma, catalog number: 1.09303.0100)
2. Conical tubes 15 mL (VWR, catalog number: 89039-664)
3. Conical tubes 50 mL (VWR, catalog number: 89039-656)
4. Cotton wool (any reputable vendor)
5. Microcentrifuge tubes 1.5 mL (VWR, catalog number: 20170-038)
6. Microcentrifuge tubes 2 mL (VWR, catalog number: 20170-022)
7. Needles (BD PrecisionGlide, catalog number: 305167)
8. Nitrogen, compressed (Airgas, catalog number: NI UHP300)
9. Oil bath with mineral oil (Fisher Scientific, catalog number: O122-1)
10. Parafilm (Millipore Sigma, catalog number: P7793)
11. Pasteur pipettes (Millipore Sigma, catalog number: Z627992)
12. Pasteur pipette bulbs (Millipore Sigma, catalog number: Z111597)
13. Polypropylene syringes for peptide synthesis equipped with a filter and cap (Peptideweb, catalog number: PPV012L and PPVLC1)
14. Sterile, filtered, low-retention pipette tips: 1,000 μL (VWR, catalog number: 76322-154), 200 μL (VWR, catalog number: 76322-150), 10 μL (VWR, catalog number: 76322-528), 20 μL (VWR, catalog number: 76322-134), 2 μL (VWR, catalog number: 76327-214)
15. Syringes: 50 mL (BD, catalog number: 309654), 20 mL (Chemglass, catalog number: CG-3080-08), 10 mL (Chemglass, catalog number: CG-3080-06), 5 mL (Chemglass, catalog number: CG-3080-04), 1 mL (Chemglass, catalog number: CG-3080-01)
16. Test tubes (Millipore Sigma, catalog number: Z740988)
17. Weighing paper (Fisher Scientific, catalog number: 09-898-12A)
Equipment
1. Set of adjustable micropipettes covering a range of 0.1–1,000 μL (e.g., 0.1–2 μL, 2–20 μL, 20–200 μL, 100–1,000 μL from any reputable vendor)
2. Alarm timer (Santa Cruz, catalog number: sc-201632)
3. Analytical balance (Denver Instrument, catalog number: TB-224)
4. Centrifuge and rotor for 15–50 mL conical tubes (Sorvall Instruments, RC-5B Refrigerated Superspeed Centrifuge; Piramoon Technologies, Inc., FIBERLite F15-8x50c Fixed Angle Rotor)
5. Compressed air line (integrated with laboratory fume hood system)
6. Cuvette for spectrophotometric measurement, lightpath 10 mm (Fisher Scientific, catalog number: 14-385-914B)
7. Freezer (-20 °C, any reputable vendor)
8. Glass bottles with a cap: 1 oz (ULINE, catalog number: S-20888), 2 oz (ULINE, catalog number: S-20889), 6 oz (ULINE, catalog number: S-24699), 8 oz (ULINE, catalog number: S-23396)
9. Glass vial 2 mL with a cap (Agilent, catalog numbers: 5182-0716 and 5182-0717)
10. HPLC system equipped with a UV-vis detector (Agilent Technologies, model: 1260 Infinity)
11. HPLC analytical column 100-5-C18, 250 mm × 4.6 mm (Kromasil, catalog number: K08670357)
12. HPLC semipreparative column 100-5-C18, 250 mm × 10 mm (Kromasil, catalog number: K08670648)
13. Laboratory glassware: glass adapter (Chemglass, catalog number: CG-1014-01) glass column (Chemglass, catalog number: CG-1188-10), glass stopper (Chemglass, catalog number: CG-3000-14), round bottom flask 5 mL (Chemglass, catalog number: CG-1506-80), round bottom flask 100 mL (Chemglass, catalog number: CG-1506-05), round bottom flask 250 mL (Chemglass, catalog number: CG-1506-17), Schlenk line (Chemglass, catalog number: AF-0451), Schlenk tube (Chemglass, catalog number: AF-0537-01)
14. Magnetic stirrer with hot plate (IKA, model: C-MAG HS 7)
15. Magnetic stirring bar (Chemglass, model: CG-2005-30)
16. Mini centrifuge (Benchmark, model: C1008-C)
17. Peptide shaker (Peptideweb, model: LPS360PRO)
18. Refrigerator (-4 °C, any reputable vendor)
19. Rotary evaporator (BUCHI, model: Rotavapor R-300)
20. Spectrophotometer UV-Vis (Agilent, model: Cary 60 G6860A)
21. Stainless steel laboratory spatula for weighing solid reagents (any reputable vendor)
22. Test tube rack (VWR, catalog number: 89215-778)
23. Vacuum pump (Labconoco, model: 117 A65312906)
24. Vortex mixer (Fisher Scientific, model: 02215370)
Procedure
文章信息
稿件历史记录
提交日期: Jun 5, 2025
接收日期: Aug 19, 2025
在线发布日期: Sep 9, 2025
出版日期: May 5, 2026
版权信息
© 2026 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Wierzba, A. J., Richards, E. M., Lennon, S. R., Batey, R. T. and Palmer, A. E. (2026). Enhancement of RNA Imaging Platforms by the Use of Peptide Nucleic Acid-Based Linkers. Bio-protocol 16(9): e5453. DOI: 10.21769/BioProtoc.5453.
分类
生物化学 > RNA
分子生物学 > RNA > RNA定位
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link












