- EN - English
- CN - 中文
Efficient Gene Knockdown in Adult Zebrafish Retina by Intravitreal Injection
玻璃体腔注射实现成年斑马鱼视网膜的高效基因敲低
发布: 2025年09月05日第15卷第17期 DOI: 10.21769/BioProtoc.5436 浏览次数: 1382
评审: Alessandro DidonnaAnonymous reviewer(s)
Abstract
High-throughput sequencing has created a tremendous amount of information about the genes expressed in various cell types and tissues throughout the body. As such, there is a need for a quick and effective method to knock down genes of interest in order to investigate their roles. While there are many approaches for this in mammalian models, there are limited ways to knock down genes of interest in adult zebrafish. Unlike mammals, zebrafish have the natural ability to regenerate their neurons after injury or disease is detected, making them a staple in regenerative studies. Unfortunately, current approaches for gene knockdown in the retina of adult zebrafish are costly and provide a barrier for many scientists. We provide two cost-effective approaches for targeted gene knockdowns in adult zebrafish retinas. We describe this approach through the use of Vivo-morpholinos and lipid-encapsulated siRNAs that target the expression of the proliferating cell nuclear antigen (PCNA) gene in adult zebrafish. We also describe how to collect and process retina samples for downstream immunohistochemistry, imaging, and quantification. Overall, this protocol will provide researchers with a straightforward, cheap, and effective method to perform targeted gene knockdowns in adult zebrafish retinas.
Key features
• This protocol provides researchers with an approach to knock down genes of interest in adult zebrafish retina without the use of electroporation.
• This protocol can be performed without causing an acute damage response in the retina.
• This protocol allows targeting of genes in both proliferating cells and terminally differentiated cells.
• This protocol allows the retina to be collected and processed for further downstream analysis.
Keywords: Gene knockdowns (基因敲低)Graphical overview
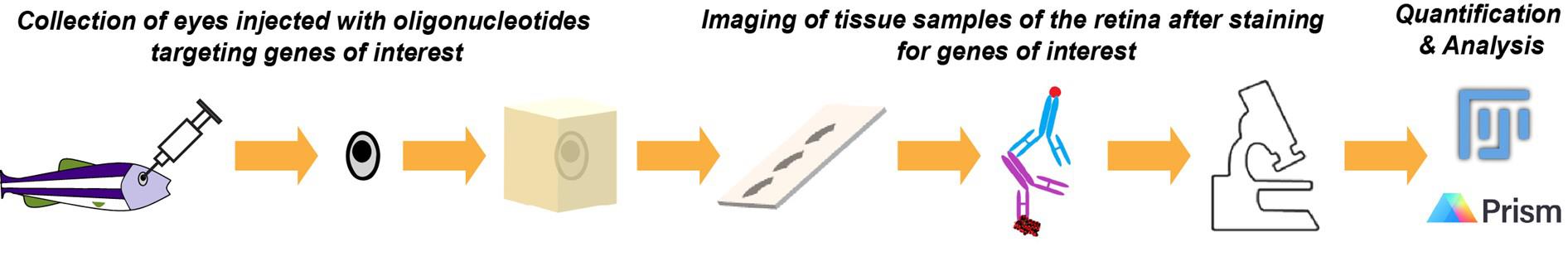
Graphical abstract illustrating the procedures required to knock down and validate the knockdown of genes of interest
Background
Ease of access to high-throughput sequencing in recent years has provided researchers with a plethora of data to mine. Whether they are investigating genes in relation to chronic disease, developmental defects, or even evolution, cell atlases have come to provide researchers with a multitude of genes to investigate. Unfortunately, limited resources and funding for most labs requires a quick and efficient method to screen genes of interest through gene knockdowns. While researchers can readily utilize a diverse array of viral vectors, CRISPR-Cas systems, and nanoparticles to target and knock down genes of interest in mammalian model systems, adult zebrafish models still lack a quick and effective method for targeted gene knockdown [1–3]. Unlike mammalian models, zebrafish have the remarkable ability to regenerate their fins, cardiomyocytes, and even neurons throughout their entire lifespans. As such, many researchers are trying to identify the network of genes responsible for regeneration so they may translate these findings to mammalian models. In adult zebrafish retina, targeted gene knockdowns have been achieved through the electroporation of morpholino oligonucleotides into the retina [4–6]. Many zebrafish labs do not have an electroporation device readily available, which may cost in excess of US$16,000, and must rely on alternative strategies. One alternative approach is the use of Vivo-morpholino oligonucleotides. Vivo-morpholinos are designed so that a morpholino oligomer is linked to a molecular transporter with eight guanidinium head groups and does not require electroporation for successful penetrance of cells [7]. While several studies have successfully used Vivo-morpholinos for targeted gene knockdowns in adult zebrafish, none of these studies worked on the retina [8–13]. Another popular strategy for targeted gene knockdowns is through the use of siRNAs. SiRNAs degrade mRNA strands of interest by utilizing the endogenous RISC cascade [14,15]. One limitation of siRNAs for in vivo experiments is their successful delivery to the cells of interest. One strategy to overcome this limitation is through encapsulation in lipid particles; however, the efficiency of these particles varies from species to species and tissue to tissue. In this study, we describe a quick and easy method to effectively knock down genes of interest in adult zebrafish retina through the use of either Vivo-morpholinos or lipid-encapsulated siRNAs. We provide detailed reagents and a step-by-step protocol on how to prepare the solutions needed to knock down PCNA in adult zebrafish retina. The model system used in this study is a transgenic zebrafish expressing a Flag-tagged mouse rhodopsin harboring the P23H mutation in rod photoreceptors. This results in rapid degeneration of rods, producing a model of Retinitis Pigmentosa [16]. Because of the robust regenerative capabilities of the zebrafish, this model displays continuous regeneration of degenerated rods, with rapid proliferation of retinal progenitor cells that express PCNA. In this protocol, we will explain how to process samples of the retina and quantify the expression of PCNA between treatment and control conditions. We show cost-effective strategies that will allow researchers to quickly and efficiently target and knock down genes in the retina of adult zebrafish.
Materials and reagents
Biological materials
1. Adult P23H rhodopsin transgenic zebrafish [generated in-house, RRID: uth4tg(AB); ZFIN ID: ZDB-FISH-220323-6]
Reagents
1. 0.01 M phosphate-buffered saline (PBS) (Millipore-Sigma, catalog number: P3813)
2. UltraPure DNase/RNase-free distilled water (Invitrogen, catalog number: 10977015)
3. DDH2O (made in-house)
4. Tricaine/MS222 (Syndel, catalog number: SYNC-M-GR-US02)
5. Tris (Fisher, catalog number: BP152-500)
6. 32% Formaldehyde (PFA) (Electron Microscopy Sciences, catalog number: 15714-S)
7. 190 Proof (95%) ethanol (ETOH) (Fisher, catalog number: 04-355-454)
8. Sucrose (Millipore-Sigma, catalog number: S0389-500G)
9. Tissue-Tek CRYO-O.C.T. compound (Fisher Scientific, catalog number: 14-373-65)
10. Dry ice
11. Triton X-100 (Sigma-Aldrich, catalog number: X100-1L)
12. Normal donkey serum (NDS) (Jackson ImmunoResearch, catalog number: 017-000-121)
13. Flag-DDK primary antibody (Origene, catalog number: TA50011; RRID: AB_2622345)
14. PCNA primary antibody (Abcam, catalog number: Ab18197; RRID: AB_444313)
15. Cy3 secondary antibody (Jackson ImmunoResearch, catalog number: 115-165-206; RRID: AB_2338695)
16. Alexa Fluor 488 secondary antibody (Jackson ImmunoResearch, catalog number: 711-545-152; RRID: AB_2313584)
17. Vectashield with DAPI (Vector Laboratories, catalog number: H-1000)
18. Altogen Nanoparticle In Vivo Transfection kit (Altogen, catalog number: 5030)
19. Zebrafish water (reverse osmosis-purified water adjusted to 700 nS conductivity with Instant Ocean sea salt mix and pH 7.0 with sodium bicarbonate)
20. Custom translation blocking Vivo-morpholino targeting PCNA (Gene Tools, sequence: TTTCTTAGTTTGGAGTAGGAGGAAC)
21. Standard negative control Vivo-morpholino (Gene Tools, sequence: CCTCTTACCTCAGTTACAATTTATA)
22. Custom siRNA targeting PCNA #1 (IDT, target sequence: GTCCAAGACGGTCACACTTAGCATG)
23. Custom siRNA targeting PCNA #2 (IDT, target sequence: AAGACGGTCACACTTAGCATGTCCG)
24. Custom siRNA targeting EGFP (negative control) (IDT, target sequence: GCATGCATCTCAATTAGTCAGCAAC)
Solutions
1. Tricaine stock solution (4%) (see Recipes)
2. Anesthesia solution (see Recipes)
3. Euthanasia solution (see Recipes)
4. Fixation solution (see Recipes)
5. Blocking buffer (see Recipes)
6. Primary antibody solution (see Recipes)
7. Secondary antibody solution (see Recipes)
8. Vivo-morpholino solution (see Recipes)
9. siRNA solution (see Recipes)
10. 25% sucrose solution (see Recipes)
11. 10% Triton X-100 (see Recipes)
Recipes
1. Tricaine stock solution (4%)
Add 4.0 g of Tricaine/MS222 to 97 mL of ddH2O. Adjust pH to 7.0 with 1 M Tris base. Top off to 100 mL and store in 10 mL aliquots frozen at -20 °C.
2. Anesthesia solution
Each adult zebrafish must be anesthetized with Tricaine/MS222 before performing any injections. To check that the zebrafish is fully anesthetized, the experimenter should be able to use a plastic spoon to pick up the zebrafish without any response. This typically takes about 3–5 min. Anesthesia solution should be made fresh for each experiment.
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Tricaine stock solution | 0.02% | 0.5 mL |
| Zebrafish water | - | 100 mL |
3. Euthanasia solution
Zebrafish should be euthanized immediately prior to the collection of samples for downstream analysis. Euthanasia solution should be made fresh for each experiment.
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Tricaine stock solution | 0.04% | 1 mL |
| Zebrafish water | - | 100 mL |
4. Fixation solution
Fixation solution should be made fresh for each experiment. It consists of a 9:1 95% EtOH to 32% PFA ratio.
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Ethanol | 85.5% | 9 mL |
| PFA | 3.2% | 1 mL |
5. Blocking buffer
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| 10% Triton X-100 | 0.3% | 9 μL |
| NDS | 5% | 15 μL |
| 1× PBS | - | 276 μL |
6. Primary antibody solution
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| 10% Triton X-100 | 0.3% | 9 μL |
| NDS | 5% | 15 μL |
| Flag-DDK primary antibody | 1:200 dilution | 1.5 μL |
| PCNA primary antibody | 1:100 dilution | 3 μL |
| 1× PBS | - | 271.5 μL |
7. Secondary antibody solution
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| 10% Triton X-100 | 0.3% | 9 μL |
| NDS | 5% | 15 μL |
| Cy3 secondary antibody | 1:500 dilution | 0.6 μL |
| Alexa 488 fluor secondary antibody | 1:500 dilution | 0.6 μL |
| 1× PBS | - | 274.8 μL |
8. Vivo-morpholino solution
Vivo-morpholinos are delivered as powders and should be stored in the dark and at room temperature until ready to use. When making the stock solution, dissolve the Vivo-morpholino in ultraclean water to 400 μM. This concentration may need to be adjusted depending on oligo properties; follow the manufacturer’s instructions. The manufacturer recommends storing the stock solution at room temperature. To make the working solution, dilute the stock solution with sterile-filtered PBS. Typically, Vivo-morpholinos precipitate out of the stock solution and lose efficacy within 1 month. This may vary depending on the Vivo-morpholino. Always check the stock solution for precipitates before making the working solution. The working solution should be made fresh on the day of injections. Each Vivo-morpholino will have its own unique efficacy. Be sure to use freshly prepared Vivo-morpholino when evaluating efficacy.
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Vivo-morpholino | 40 μM | 2 μL |
| 0.01 M PBS | - | 18 μL |
9. siRNA solution
Dissolve siRNA in 10 mM Tris, pH 8.0, following manufacturer’s instructions. siRNA stock is generally 225 μM, and should be stored at -80 °C. Each siRNA will have a different efficacy when optimally transfected. It is often more effective to combine two or more siRNAs to the desired target gene. The Altogen Nanoparticle reagents typically have a shelf life of 3 months when stored at 4 °C. Always check for precipitates before using.
| Reagent | Final concentration | Quantity or Volume |
| siRNA #1 | 37.5 μM | 1.5 μL |
| siRNA #2 | 37.5 μM | 1.5 μL |
| Altogen Nanoparticle In Vivo transfection reagent | - | 5 μL |
| Incubate at room temperature for 20 min. Then, add 1 μL of Altogen Nanoparticle In Vivo transfection enhancer. Incubate for 5 min at room temperature; then, it is ready to use. | ||
10. 25% sucrose solution
2.5 g of sucrose in a final volume of 10 mL of ddH2O; made fresh for each experiment.
11. 10% Triton X-100
1 mL of Triton X-100 and 9 mL of ddH2O.
Laboratory supplies
1. Petri dish (Fisher, catalog number: 08-757-100B)
2. Disposable pipettes (Fisher, catalog number: 13-711-7M)
3. 50 mL Falcon tubes (Fisher, catalog number: 14-959-49A)
4. 1.5 mL Eppendorf tubes (Fisher, catalog number: 14-222-158)
5. Paper towels (Fisher, catalog number: 06-666-32B)
6. Saphire blade (World Precision Instruments, catalog number: 504072)
7. Sterile 32-gauge blunt 5 μL NEUROS Syringes (Stoelting, catalog number: 53496)
8. 24-well plate (Fisher, catalog number: 09-761-146)
9. Embedding molds (Fisher, catalog number: 2219)
10. Microtome blades (Fisher, catalog number: 4280L)
11. Superfrost Plus Gold slides (Fisher, catalog number: 1518848)
12. Hydrophobic barrier PAP pen (Cole-Parmer, catalog number: 7595553)
13. Coplin jars (Electron Microscopy Sciences, catalog number: 70315)
14. Coverslips (Fisher, catalog number: 15-541-000)
15. Nail polish (Fisher, catalog number: 50-949-071)
16. Kimwipes (Fisher, catalog number: 06-666)
Equipment
1. Mechanical micromanipulator (Sutter Instruments, model: MM-33)
2. Stereomicroscope (Olympus, model: SZ51)
3. Cryostat (Leica, model: CM1950)
4. Confocal microscope (Zeiss, model: LSM 800)
5. PC (Windows 11)
Software and datasets
1. FIJI (1.54F; free): https://imagej.net/software/fiji/
2. Zen (Black; free): https://www.zeiss.com/microscopy/us/products/software/zeiss-zen.htmL
3. Excel (2016; requires a license but Google Sheets can be used as a free alternative)
4. GraphPad Prism 9 (9.5.0; requires a license)
Procedure
文章信息
稿件历史记录
提交日期: Apr 6, 2025
接收日期: Jul 22, 2025
在线发布日期: Aug 12, 2025
出版日期: Sep 5, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Shihabeddin, E., Santhanam, A., Tetenborg, S., Aronowitz, A. L. and O`Brien, J. (2025). Efficient Gene Knockdown in Adult Zebrafish Retina by Intravitreal Injection. Bio-protocol 15(17): e5436. DOI: 10.21769/BioProtoc.5436.
分类
神经科学 > 感觉和运动系统 > 视网膜
细胞生物学 > 细胞工程
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link