- EN - English
- CN - 中文
Simultaneous RNA Fluorescent In Situ Hybridization and Immunofluorescent Staining of Mouse Muscle Stem Cells on Fresh Frozen Skeletal Muscle Sections
新鲜冷冻骨骼肌切片上小鼠肌肉干细胞的RNA荧光原位杂交与免疫荧光染色同步检测
(§ Technical contact) 发布: 2025年09月05日第15卷第17期 DOI: 10.21769/BioProtoc.5435 浏览次数: 1913
评审: Sébastien GillotinAnonymous reviewer(s)
Abstract
Adult muscle stem cells (MuSCs) are the key cellular source for regenerating skeletal muscle in vertebrates. MuSCs are typically identified in skeletal muscle by the expression of the paired box protein 7 (PAX7) protein. Here, we developed a combined RNA fluorescent in situ hybridization (FISH) using RNAscope technology and an immunofluorescence (IF) protocol for the simultaneous detection of Pax7 mRNA and PAX7 protein in individual MuSCs in vivo. Interestingly, we show that while most PAX7+ (protein) MuSCs express Pax7 mRNA, there is a subset of Pax7+ (mRNA) cells that do not express PAX7 protein. Altogether, we developed a combined FISH/IF protocol that allows for the co-detection of mRNA and protein in MuSCs in vivo, a strategy that can be applied to any target gene. The functional significance of the Pax7-expressing subset of cells lacking PAX7 protein prior to injury remains unknown.
Key features
• Extensive step-by-step details for an optimized protocol combining traditional immunofluorescence with RNA fluorescent in situ hybridization (FISH) using ACDBio’s RNAscope technology.
• Allows for the co-detection of protein and mRNA in muscle stem cells (MuSCs) in mouse skeletal muscle tissue in vivo in ~2 days.
• Validation of our protocol uncovers a subset of cells expressing Pax7 mRNA but not PAX7 protein.
Keywords: Immunofluorescence (IF) (免疫荧光(IF))Graphical overview
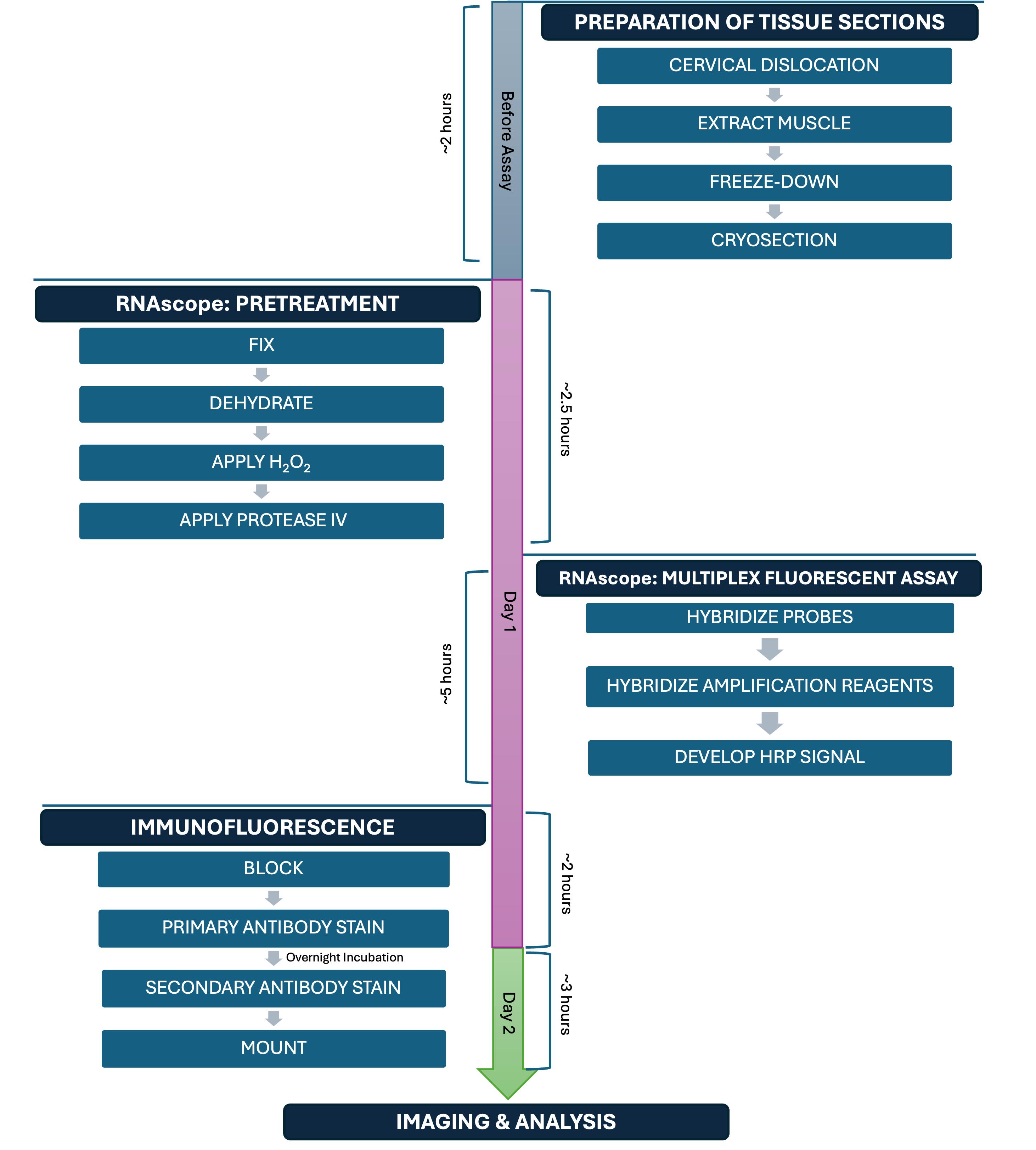
Background
Muscle satellite cells (MuSCs) are a stem cell population responsible for regenerating skeletal muscle in vertebrate animals [1–6]. The paired box 7 (PAX7) protein is the canonical marker gene that is uniquely expressed by adult MuSCs in mammals [5]. The most common way of visualizing and quantifying PAX7-expressing MuSCs in skeletal muscle tissue before and after injury, in pathogenic states, or during aging in vivo is using immunofluorescence (IF) for PAX7 protein or with transgenic mice expressing a fluorescent protein using the Pax7 promoter (i.e., a fluorescent marker as a surrogate for PAX7 expression) [7].
A major limitation in studying MuSC fate decisions in mammalian tissue sections in vivo is the challenge in finding validated antibodies for performing a co-stain between PAX7 and your favorite protein of interest. While several good PAX7 antibodies exist, it can be difficult to find IF conditions that preserve PAX7 while also allowing for the detection of other antigens that indicate a change in differentiation, cell survival, cell proliferation, or other cell state changes. One potential way to address this need is to perform simultaneous detection of (1) PAX7 protein with IF and (2) RNA fluorescent in situ hybridization (FISH) of your favorite gene. Several companies have developed protocols for FISH, including ACDBio (RNAscope), Biosearch Technologies (Stellaris), and ViewRNA (Thermo Fisher Scientific), which vary in terms of probe chemistry, reagents used, and workflow.
Specifically, ACDBio commercialized a new technology called RNAscope [8], which is a proprietary type of RNA FISH that has gained popularity over the years. RNAscope is a technique that involves six major steps: 1) preparing your tissue sample; 2) pretreatment; 3) probe hybridization; 4) amplification steps; 5) labeling of sites where the probe is binding the target; and 6) detection of the signal. ACDBio established a streamlined RNAscope/IF protocol; however, there are several steps that need to be optimized for every RNA target and cell or tissue type. Indeed, a recent study developed an optimized RNAscope protocol for detecting protein and RNA simultaneously in single myofibers in culture ex vivo [9].
Here, we present a detailed RNAscope/IF protocol for performing simultaneous detection of mRNA and protein, using Pax7 mRNA and PAX7 protein in MuSCs from uninjured skeletal muscle sections as an example. Overall, our protocol enables multiplexed analysis via the visualization of mRNA and protein targets within the same tissue section. Additionally, while performing our validation studies for this protocol, we discovered that while the majority of PAX7+ (protein) cells express Pax7 mRNA, about 17% of cells that express Pax7 mRNA have no detectable PAX7 protein. These data suggest that standard IF-based methods for detecting PAX7-positive cells alone likely miss a subset of Pax7-expressing cells that have not yet accumulated protein and whose functional significance remains unknown.
Materials and reagents
Biological materials
1. Mice, C57BL/6J (Mus musculus) (The Jackson Laboratory, catalog number: 000664)
Reagents
1. Bovine serum albumin (BSA) (Sigma-Aldrich, catalog number: A9418-50G)
2. Normal goat serum (NGS) (VWR, catalog number: 102643-594)
3. Paraformaldehyde (PFA) (Electron Microscopy Sciences, catalog number: 15714-S)
4. M.O.M.® blocking reagent (Mouse on Mouse) (Vector Laboratories, catalog number: MKB-2213-1)
5. Phosphate-buffered saline (PBS), 1× without calcium and magnesium (VWR, catalog number: 45000-446)
6. Hoechst 33342 solution, 20 mM (Life Technologies, catalog number: 62249)
7. Triton X-100 (VWR, catalog number: AAJ66624-AP)
8. Tween-20 (Fisher Scientific, catalog number: BP337-100)
9. Anti-PAX7 (Developmental Studies Hybridoma Bank, DSHB, catalog number: PAX7, RRID: AB_528428); see General note 1 for how we prepared the DSHB PAX7 antibody
10. Goat anti-mouse IgG1 cross-adsorbed secondary antibody, Alexa FluorTM 555 (Thermo Fisher Scientific, catalog number: A21127)
11. Reagent-grade 100% alcohol for histology (VWR, catalog number: 89370-084)
12. RNAscope® Probe, Mm-Pax7, Mus musculus paired box gene 7 (Pax7), mRNA (Advanced Cell Diagnostics, catalog number: 314181)
Caution: This reagent contains formamide, which is carcinogenic.
13. RNAscope® 3-plex positive control probe, Mm, RNAscope® mouse positive control probe for RNAscope® multiplex fluorescent assay, UBC, C3 channel (Advanced Cell Diagnostics, catalog number: 320881)
Caution: This reagent contains formamide, which is carcinogenic.
14. RNAscope® 3-plex negative control probe, RNAscope® negative control probe C1 channel dapB (of Bacillus subtilis strain) for RNAscope® multiplex fluorescent assay (Advanced Cell Diagnostics, catalog number: 320871)
Caution: This reagent contains formamide, which is carcinogenic.
15. TSA VividTM Fluorophore kit 650 (Bio-Techne, catalog number: 7527)
16. Nail polish, clear (Electron Microscopy Sciences, catalog number: 72180)
17. RNAscope® Multiplex Fluorescent Reagent kit v2 (Advanced Cell Diagnostics, catalog number: 323100). From this kit, only the reagents listed in Table 1 were used.
Table 1. Key kit reagents for RNAscope
| Sub-kit | Component |
| RNAscope® H2O2 & Protease reagents (catalog number: 322281) | RNAscope® hydrogen peroxide |
| RNAscope® protease IV | |
| RNAscope® Multiplex Fluorescent Detection Reagents V2 (catalog number: 323110) | RNAscope® Multiplex FL V2 AMP 1* |
| RNAscope® Multiplex FL V2 AMP 2* | |
| RNAscope® Multiplex FL V2 AMP 3 | |
| RNAscope® Multiplex FL V2 HRP-C1 | |
| RNAscope® Multiplex FL V2 HRP-C3 | |
| RNAscope® Multiplex FL V2 HRP blocker | |
| RNAscope® Multiplex FL V2 DAPI | |
| RNAscope® TSA Buffer Pack (catalog number: 322809) | RNAscope® Multiplex TSA buffer |
| RNAscope® Wash Buffer Reagents (catalog number: 310091) | RNAscope® wash buffer (50×) |
18. 2-Methylbutane (isopentane) (Sigma-Aldrich, catalog number: M32631-4L)
19. Tissue-Tek® O.C.T. compound (VWR, catalog number: 25608-930)
20. Vectashield mounting medium (VWR, catalog number: 101098-042)
21. Dry ice
22. Liquid nitrogen
Solutions
1. 4% PFA in 1× PBS (see Recipes)
2. 1× wash buffer (see Recipes)
3. 50% histology-grade alcohol (see Recipes)
4. 70% histology-grade alcohol (see Recipes)
5. TSA Vivid Fluorophore 650 (see Recipes)
6. PBST (see Recipes)
7. 10% M.O.M. in PBST (see Recipes)
8. Blocking buffer (see Recipes)
9. Blocking buffer (with 1% M.O.M.) (see Recipes)
10. 1:2 Anti-PAX7 primary antibody in blocking buffer (see Recipes)
11. 1:500 Alexa Fluor 555 secondary antibody in blocking buffer (see Recipes)
12. 1 μg/mL Hoechst (see Recipes)
Recipes
1. 4% PFA in 1× PBS
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| 32% PFA | 4% | 12.5 mL |
| 1× PBS | 1× | 87.5 mL |
| Total | N/A | 100 mL |
Store at 4 °C. Prepare fresh.
2. 1× wash buffer
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| 50× wash buffer | 1× | 60 mL |
| Double-distilled H2O | N/A | 2.94 L |
| Total | N/A | 3 L |
Store at room temperature (RT) for up to 1 month.
3. 50% histology-grade alcohol
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Reagent-grade 100% alcohol for histology | 50% | 50 mL |
| 1× PBS | 1× | 50 mL |
| Total | N/A | 100 mL |
Store at RT for up to 1 month.
4. 70% histology-grade alcohol
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Reagent-grade 100% alcohol for histology | 70% | 70 mL |
| 1× PBS | 1× | 30 mL |
| Total | N/A | 100 mL |
Store at RT for up to 1 month.
5. TSA Vivid Fluorophore 650
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| TSA Vivid Fluorophore | 1:1,500 | 1 μL |
| TSA buffer | N/A | 1.499 mL |
| Total | N/A | 1.5 mL |
Store at 4 °C. Prepare fresh.
6. PBST
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| 1× PBS | 1× | 299.7 mL |
| Tween-20 | 0.1% | 300 μL |
| Total | N/A | 300 mL |
Mix with a stir bar. Store at RT for up to 1 month.
7. 10% M.O.M. in PBST
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| PBST | N/A | 1.8 mL |
| M.O.M. | 10% | 200 μL |
| Total | N/A | 2 mL |
Store at 4 °C. Prepare fresh.
8. Blocking buffer
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| PBST | N/A | 9.49 mL |
| BSA | 3% | 0.3 g |
| NGS | 5% | 500 μL |
| Triton X-100 | 0.3% | 10 μL |
| Total | N/A | 10 mL |
Mix with a stir bar. Store at 4 °C. Prepare fresh.
9. Blocking buffer (with 1% M.O.M.)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Blocking buffer | N/A | 1.98 mL |
| M.O.M. | 1% | 20 μL |
| Total | N/A | 2 mL |
Store at 4 °C. Prepare fresh.
10. 1:2 Anti-PAX7 primary antibody in blocking buffer
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Anti-PAX7 | 1:2 | 750 μL |
| Blocking buffer (without M.O.M.) | N/A | 750 μL |
| Total | N/A | 1.5 mL |
Store at 4 °C. Prepare fresh.
11. 1:500 Alexa Fluor 555 secondary antibody in blocking buffer
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Fluorophore-conjugated secondary antibody | 1:500 | 3 μL |
| Blocking buffer (without M.O.M.) | N/A | 1.497 mL |
| Total | N/A | 1.5 mL |
Shield from light. Store at 4 °C. Prepare fresh.
12. 1 μg/mL Hoechst
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| PBST | N/A | 1.9838 mL |
| 1:100 Hoechst solution | 1 μg/mL | 16.2 μL |
| Total | N/A | 2 mL |
Hoechst stock used is 20 mM. Shield from light. Store at 4 °C. Prepare fresh.
Laboratory supplies
1. ImmEdgeTM hydrophobic barrier pen (VWR, catalog number: 101098-065)
2. KIMWIPESTM delicate task wipers (VWR, catalog number: 21905-049)
3. SimportTM Scientific EasyDipTM slide staining jars (Fisher Scientific, catalog number: 22-038-489)
4. SimportTM Scientific EasyDipTM slide staining rack (Fisher Scientific, catalog number: 22-038-494)
5. Fisherbrand straight broad strong tip extra-long forceps (Fisher Scientific, catalog number: 16-100-107)
6. VWR® Premium Superfrost® Plus microscope slides (VWR, catalog number: 48311-703)
7. Disposable base mold or cryomold (Electron Microscopy Sciences, catalog number: 62352-15)
8. Secured hinge microscope slide boxes (Electron Microscopy Sciences, catalog number: 71470-W)
9. VWR® precision tweezers (VWR, catalog number: 89259-984)
10. KIMAX® griffin beakers (2 L), low form, double scale, borosilicate glass, Kimble Chase (VWR, catalog number: 89000-746)
Equipment
1. VWR® SignatureTM rocking platform shaker (VWR, catalog number: 12620-906)
2. ACD HybEZTM II hybridization system
a. HybEZTM II oven (Advanced Cell Diagnostics, catalog number: 321719)
b. HybEZTM humidity control tray with lid (Advanced Cell Diagnostics, catalog number: 310012)
c. EZ-BatchTM slide holder with 20 slide capacity (Advanced Cell Diagnostics, catalog number: 310017)
d. EZ-BatchTM wash tray (Advanced Cell Diagnostics, catalog number: 310019)
3. VWR® bead bath (VWR, catalog number: 77553-322)
4. Leica CM 1860 UV cryostat
5. Zeiss LSM 800 Axio Imager.Z2 upright confocal microscope
Software and datasets
1. Fiji (ImageJ) (Version 2.14.0, National Institute of Health, https://imagej.net/)
2. Adobe Illustrator (Version 29.2, https://www.adobe.com/home)
3. GraphPad Prism (Version 10.4.1, GraphPad Software, Inc., La Jolla, CA, Alternative: Excel Version 16.0, https://www.graphpad.com/)
4. ZEN (blue edition) (Version 3.4, https://www.zeiss.com/corporate/en/home.html)
Procedure
文章信息
稿件历史记录
提交日期: May 16, 2025
接收日期: Jul 17, 2025
在线发布日期: Aug 11, 2025
出版日期: Sep 5, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/).
如何引用
Lakkundi, V. R., Perez, M. L., Soni, K. and Almada, A. E. (2025). Simultaneous RNA Fluorescent In Situ Hybridization and Immunofluorescent Staining of Mouse Muscle Stem Cells on Fresh Frozen Skeletal Muscle Sections. Bio-protocol 15(17): e5435. DOI: 10.21769/BioProtoc.5435.
分类
干细胞 > 成体干细胞 > 肌肉干细胞
分子生物学 > RNA > RNA 检测
细胞生物学 > 基于细胞的分析方法
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link