- EN - English
- CN - 中文
Isolation and Ex Vivo Testing of CD8+ T-Cell Division and Activation Using Mouse Splenocytes
利用小鼠脾细胞分离并离体检测CD8+ T细胞的分裂与激活
发布: 2025年08月20日第15卷第16期 DOI: 10.21769/BioProtoc.5423 浏览次数: 3845
评审: Mary Luz UribeAnonymous reviewer(s)

相关实验方案
使用康可藻红素刺激冷冻保存的猪外周单个核细胞进行增殖检测,并结合FCS ExpressTM 7.18软件分析
Marlene Bravo-Parra [...] Luis G. Giménez-Lirola
2025年06月05日 2614 阅读
外周血中细胞外囊泡的分离与分析方法:红细胞、内皮细胞及血小板来源的细胞外囊泡
Bhawani Yasassri Alvitigala [...] Lallindra Viranjan Gooneratne
2025年11月05日 1434 阅读
Abstract
This protocol describes an ex vivo co-culture method to assess CD8+ T-cell activation, proliferation, and cytotoxic potential using bulk splenocytes isolated from immunocompetent mice. Mouse splenocytes are stimulated with anti-CD3 and anti-CD28 antibodies to activate CD8+ T cells, which are then co-incubated with either cancer cells or cancer cell–derived conditioned media (CM) to evaluate tumor-driven modulation of immune cell functions. The use of unfractionated splenocytes preserves physiological cell–cell interactions, eliminating the need for exogenous interleukin (IL-2) and bypassing flow sorting, which simplifies the workflow and reduces experimental variability. CD8+ T-cell responses are measured via flow cytometry, using markers of proliferation (CFSE dilution), activation (CD69), and effector function (Granzyme B and IFNγ). Additionally, immune-mediated tumor cell death is evaluated by Annexin-V/7-AAD staining. Together, this experimental platform supports the investigation of both cell contact-dependent and contact-independent mechanisms of immune cell modulation in a cost-effective and reproducible setting.
Key features
• Enables isolation and stimulation of splenocytes to assess CD8+ T-cell responses to cancer cells or their secreted factors.
• Supports evaluation of CD8+ T-cell activation, proliferation, and effector function by flow cytometry.
• Allows functional assessment of tumor-driven suppression of T-cell activation in co-culture or conditioned media.
• Measures cancer cell death resulting from interactions with activated CD8+ T cells in splenocyte co-cultures.
Keywords: Tumor-immune cross-talk (肿瘤-免疫互作)Graphical overview
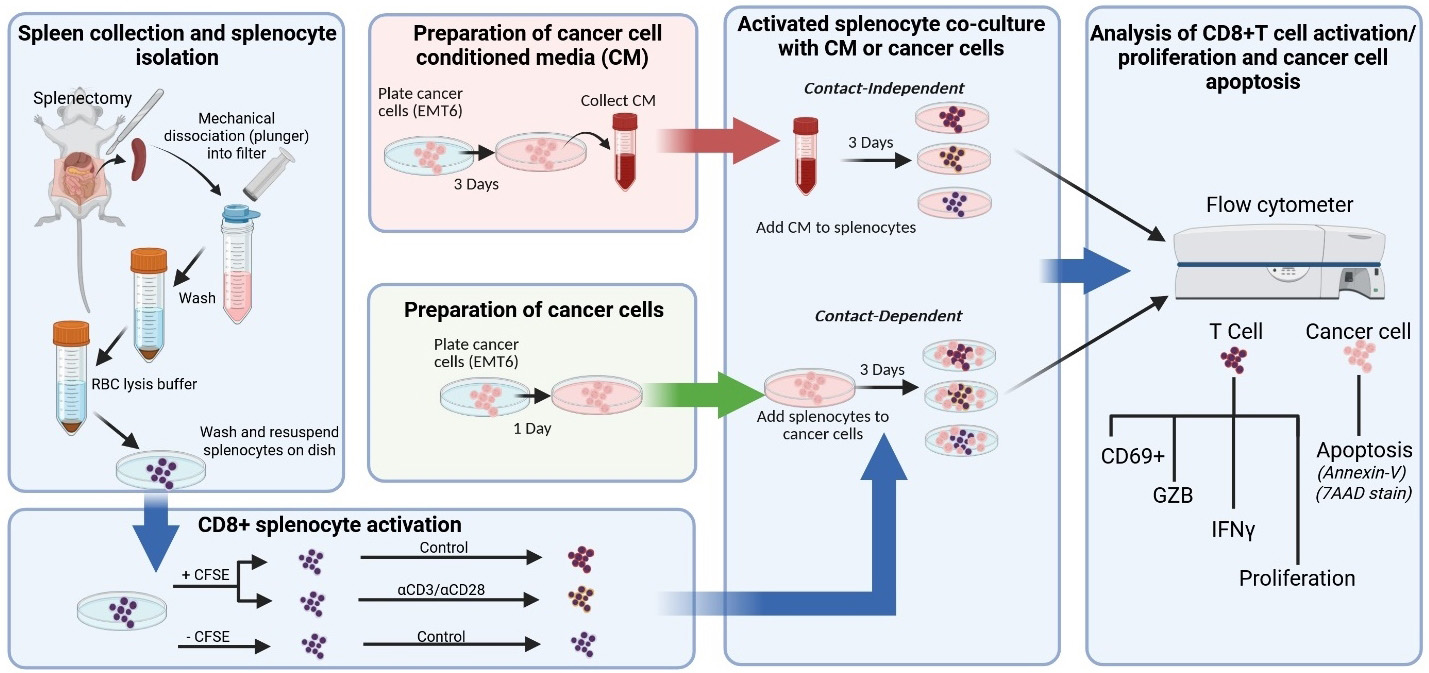
Graphical overview of key protocol steps
Background
Immunotherapies targeting T-cell functions are now approved in several tumor types [1]. This has led to a dramatic increase in the need to develop preclinical methods to identify ways to improve patient responses and evaluate drug resistance [2,3]. In vivo studies with preclinical mouse syngeneic tumor models are most commonly used for gaining functional insight into human cancer progression in an immunocompetent setting, but they are limited by costs and variability. Testing of in vivo–derived immune components in ex vivo settings represents a way to mimic the complexity of tumor and immune cell functions and interactions with improved reproducibility/rigor, reduced cost, and improved control over experimental variables.
Here, we describe a protocol to evaluate the functional interaction between activated CD8+ T cells within mouse splenocyte populations when cultured with cancer cells or cancer cell conditioned media (CM), a technique that allows comparison of cell contact-dependent and contact-independent mechanisms of immune cell modulation [4,5]. The use of bulk mouse splenocytes eliminates the need for stimulation with interleukin-2 (IL-2) and enrichment techniques to isolate pure T-cell populations. IL-2 is a cytokine released by surrounding immune cells (e.g., CD4+ T cells or CD8+ T cells themselves) in secondary lymphoid organs or the tumor microenvironment that promotes expansion of T cells following CD3/T-cell receptor (TCR) and CD28/CD80 activation signaling [6,7]. Endogenous IL-2 produced by bulk splenocytes may better mimic cytokine concentrations present within the secondary lymphoid organs following antigen stimulation by antigen-presenting cells (APCs) [8] and may control for variability across studies that use different IL-2 stimulation concentrations in conventional ex vivo CD8 stimulation/activation protocols [6,7].
Importantly, the protocol described here examines antigen-independent CD8+ T-cell responses that may be advantageous for studying scientific questions involving immune suppressive secreted factors derived from cancer cells and cell surface proteins that can induce T-cell suppression. Ex vivo experiments specifically assessing antigen presentation may also find some aspects of this protocol (e.g., isolation of splenocytes and flow cytometry analysis of T-cell proliferation and activation markers) helpful for guidance in similar steps. We describe the protocol below using EMT6 parental/drug-resistant (DR) murine breast cancer cells and splenocytes derived from the corresponding allogenic BALB/c mice as an example. Alternatively, other mouse cancer cell lines and mouse strains (e.g., commonly used strains including FVB or C57BL/6) can be substituted in this protocol for broader applications. Other critical biological variables, such as age [9] and biological sex [10], which have profound effects on innate and adaptive immune responses and responses to cancer immunotherapy, can be applied to this protocol as well.
Our protocol design allows distinguishing between contact-dependent and contact-independent mechanisms of generalized CD8+ T-cell responses, including the identification of specific soluble secreted factors with strong immune modulating impact [4,5]. While this protocol is broadly applicable to various research contexts, here we compared CD8+ T-cell responses using cancer cells that are naïve or resistant to immunotherapy [4,5]. Together, this method allows elucidation of tumor-immune cell dynamics related to mechanisms of therapeutic resistance in a reproducible and cost-effective setting.
Materials and reagents
Biological materials
1. BALB/cAnNCrl mice (Charles River Laboratory, strain code: 028) as a source for spleens
2. EMT6 murine breast carcinoma cells (gifted by Laboratory of A.Gudkov)
3. EMT6 drug-resistant cell line (generated in [4])
Reagents
1. 1× Dulbecco’s phosphate buffer saline (DPBS) without Ca2+ and Mg2+, pH 7.4 (Corning, catalog number: 21-040-CV)
2. RPMI 1640 media with L-glutamine (Corning, catalog number: 10-040-CV)
3. Heat-inactivated fetal bovine serum (HI-FBS) (Gibco, catalog number: 10437028)
4. Penicillin-Streptomycin (Pen/Strep) (Corning, catalog number: 30-001-CI)
5. Sodium pyruvate (Gibco, catalog number: 11360070)
6. Non-essential amino acids (Gibco, catalog number: 11140050)
7. Beta-mercaptoethanol (β-me) (Gibco, catalog number: 21985023)
8. Red blood cell (RBC) lysis buffer (10×) (BioLegend, catalog number: 420301)
9. Accutase (BioLegend, catalog number: 423201)
10. 0.25% trypsin (Corning, catalog number: 25-053-CI)
11. Trypan blue solution, 0.4% (Corning, catalog number: 25-900-CI)
12. T-cell activation antibodies (see Table 1)
13. Flow cytometry staining antibodies (see Table 1)
Table 1. General antibodies used in the protocol
| Antibody | Purpose | Host | Company | Catalog number |
|---|---|---|---|---|
| Anti-mouse CD3 (clone 17-A2) | Cell activation | Rat | BioLegend | 100202 |
| Anti-mouse CD28 (clone 37.51) | Cell activation | Syrian hamster | BioLegend | 102102 |
| Anti-mouse CD16/CD32 TruStain FcX | Surface cell staining | Rat | BioLegend | 101319 |
| Anti-mouse CD45 (clone 30-F11); Alexa Fluor 700 conjugated | Surface cell staining | Rat | BioLegend | 103128 |
| Anti-mouse CD8b.2 (clone 53-5.8); PE-Cy7 conjugated | Surface cell staining | Rat | BioLegend | 140416 |
| Anti-mouse CD69 (clone H1.2F3); APC conjugated | Surface cell staining | Armenian hamster | BioLegend | 104514 |
| Anti-mouse/human Granzyme B (clone GB11); Pacific Blue conjugated | Intracellular cell staining | Rat | BioLegend | 515408 |
| Anti-mouse IFNγ (clone XMG1.2); PE conjugated | Intracellular cell staining | Rat | BioLegend | 505808 |
14. Fixation buffer (BioLegend, catalog number: 420801)
15. Intracellular staining permeabilization wash buffer (10×) (BioLegend, catalog number: 421002)
16. Carboxyfluorescein succinimidyl ester (CFSE) Cell Division Tracker kit (BioLegend, catalog number: 423801)
17. Sodium azide (NaN3) (Sigma-Aldrich, catalog number: S-2002)
18. Annexin-V APC (BioLegend, catalog number: 640941)
19. 7-AAD viability staining solution (BioLegend, catalog number: 420404)
20. Annexin-V binding buffer (BioLegend, catalog number: 422201)
21. Cell activation cocktail [containing phorbol 12-myristate 13-acetate (PMA) Ionomycin and Brefeldin A1] (BioLegend, catalog number: 423303)
22. Staurosporine (Fisher Scientific, catalog number: AAJ62837MCR)
Solutions
1. Spleen collection solution (see Recipes)
2. Splenocyte wash solution (see Recipes)
3. Splenocyte complete RPMI (see Recipes)
4. RBC lysis buffer (see Recipes)
5. Cancer (EMT6) cell growth media (see Recipes)
6. Flow cytometry staining wash buffer (See Recipes)
7. Intracellular permeabilization and wash buffer (see Recipes)
Recipes
1. Spleen collection solution (5 mL for 1 spleen)
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| DPBS (1×) | n/a | 4.60 mL |
| HI-FBS | 5% | 50 μL |
| Pen/Strep (100,000 UI/mL) | 1,000 UI/mL | 5 μL |
| Total | 5 mL |
2. Splenocyte wash solution (20 mL for 1 spleen)
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| DPBS (1×) | n/a | 19.6 mL |
| HI-FBS | 2% | 0.4 mL |
| Total | 20 mL |
3. Splenocyte complete RPMI (500 mL)
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| RPMI 1640 | n/a | 435 mL |
| HI-FBS | 10% | 50 mL |
| Pen/Strep (100,000 UI/mL) | 1,000 UI/mL | 5 mL |
| Non-essential amino acids (100×) | 1× | 5 mL |
| Sodium pyruvate (100×) | 1× | 5 mL |
| β-me (55 mM in DPBS) | 0.1% | 0.5 mL |
| Total | 500.5 mL |
Store at 4 °C for a maximum of 6–8 weeks.
4. RBC lysis buffer (3 mL per 1 spleen)
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| RBC lysis buffer (10×) | 1× | 0.3 mL |
| diH2O | n/a | 2.7 mL |
| Total | 3 mL |
Make fresh for every dissociation. Keep sterile and use sterile deionized H2O (diH2O).
5. Cancer (EMT6) cell growth media (500 mL)
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| RPMI 1640 | n/a | 475 mL |
| HI-FBS | 5% | 25 mL |
| Total | 500 mL |
Store at 4 °C for a maximum of 6–8 weeks.
Note: Antibiotics such as Pen/Strep can be optionally added at a final concentration of 1,000 UI/mL when subculturing cancer cells prior to co-culture with isolated splenocytes. Use of antibiotics should be kept consistent with the individual lab’s standard operating procedures. The volume of RPMI 1640 will need to be adjusted accordingly.
6. Flow cytometry staining wash (FACS) buffer (500 mL)
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| DPBS (1×) | n/a | 490 mL |
| HI-FBS | 2% | 10 mL |
| NaN3 | 0.1% | 0.5 mL |
| Total | 500.5 mL |
Critical: Wear necessary personal protective equipment (PPE) and adhere to handling precautions as indicated in the material safety data sheet (MSDS) of sodium azide (NaN3).
7. Intracellular permeabilization and wash buffer (100 mL)
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Intracellular permeabilization buffer (10×) | 1× | 10 mL |
| diH2O | n/a | 90 mL |
| Total | 100 mL |
Laboratory supplies
1. Pipettes
2. Pipette tips
3. Multichannel pipettes
4. Serological pipettes
5. Serological pipettor
6. 15 mL conical tubes (Fisher Scientific, catalog number: 601052)
7. 50 mL conical tubes (Fisher Scientific, catalog number: 602052)
8. 2 mL microcentrifuge tubes (Laboratory Product Sales Inc., catalog number: L211574)
9. 5 mL Falcon round-bottom polystyrene FACS tubes (Fisher Scientific, catalog number: 14-959-2A)
10. Bio Lite 10 cm cell culture treated plates (Fisher Scientific, catalog number: 12-556-002)
11. Corning 96-well clear round-bottom culture plates (non-treated) (Fisher Scientific, catalog number: 07-200-760)
12. Bio Lite 24-well cell culture treated plates (Fisher Scientific, catalog number: 12-556-006)
13. BD Disposable 3 mL syringes with Luer-Lok tips (Fisher Scientific, catalog number: 14-823-435)
14. Cell strainer 70 μm (Fisher Scientific, catalog number:22-363-548)
15. Corning 0.2 μm syringe filter (Fisher Scientific, catalog number: 09-754-23)
Equipment
1. Surgical scissors
2. Curved forceps
3. Biological safety cabinet (Biosafety Level 2)
4. 37 °C, 5% CO2 cell culture incubator (Thermo, model: Heraeus HeraCell 150)
5. Temperature-regulated water bath
6. -20 °C freezer (Crosley, model: WCF15/F)
7. -80 °C freezer (Sanyo, model: MDF-U76VC)
8. Microscope (Nikon, model: Eclipse TS100)
9. Temperature-controlled centrifuge (Beckman Coulter, model: Allegra 6R)
10. TC20 automated cell counter (Bio-Rad, catalog number: 145-0101)
11. Hemocytometer (Hausser Scientific, catalog number: 1483)
12. Flow cytometer (BD Biosciences, model: LSR II)
Software and datasets
1. BD FACSDiva Software (BD Biosciences)
2. FCS Express 7 (v. 7.24; De Novo Software)
3. GraphPad (v10)
Procedure
文章信息
稿件历史记录
提交日期: May 2, 2025
接收日期: Jul 17, 2025
在线发布日期: Aug 1, 2025
出版日期: Aug 20, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Dolan, M., Shi, Y., McKenery, A., Tzetzo, S., Chida, K., Takabe, K., Abrams, S. I. and Ebos, J. M. (2025). Isolation and Ex Vivo Testing of CD8+ T-Cell Division and Activation Using Mouse Splenocytes. Bio-protocol 15(16): e5423. DOI: 10.21769/BioProtoc.5423.
分类
癌症生物学 > 肿瘤免疫学 > 免疫学试验
细胞生物学 > 基于细胞的分析方法 > 流式细胞术
免疫学 > 免疫细胞功能 > 抗原特异反应
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link