- EN - English
- CN - 中文
Intravitreal NHS-Biotin Injection and Immunohistochemistry to Label and Image Protein Transport in the Mouse Optic Nerve
玻璃体腔内NHS-生物素注射结合免疫组织化学标记与成像分析小鼠视神经中的蛋白运输
发布: 2025年08月20日第15卷第16期 DOI: 10.21769/BioProtoc.5419 浏览次数: 2682
评审: Geoffrey C. Y. LauAnonymous reviewer(s)
Abstract
The process of moving proteins and organelles along the axon is essential for neuronal survival and function, ensuring proper communication between the cell body and distant synapses. The efficient and precise delivery of proteins via axon transport is critical for processes ranging from synaptic plasticity and neurotransmission to neuronal growth and maintenance. However, the identities of all the transported proteins have only recently begun to be investigated. Retinal ganglion cells (RGCs) provide a unique opportunity for access to central nervous system (CNS) axons as the retina is located outside the brain in the eye, with long axonal projections (~1 cm in mouse) that innervate the brain. We have developed and optimized methods for unbiased in vivo protein labeling in rodent RGC somata with intravitreal N-hydroxysuccinimido (NHS)-biotin and subsequent visualization of transported proteins along the optic nerve using confocal microscopy. Here, we describe these procedures in detail.
Key features
• Expands on the intravitreal injection method presented in Schiapparelli et al. [1] and extends it to whole mount optic nerve immunolabeling.
• Unbiased in vivo labeling of proteins in the mouse central nervous system.
• Optimized mouse intravitreal injection procedure using glass needles to reduce injury and costs.
• Immunofluorescent imaging of the cleared intact optic nerve to visualize transported labeled proteins.
Keywords: Axon transport (轴突运输)Graphical overview
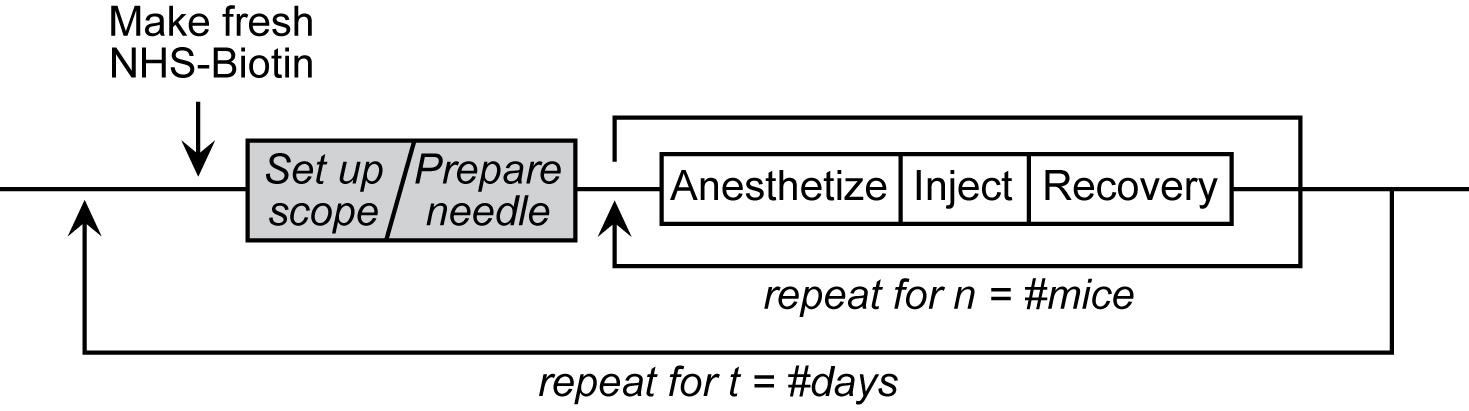
Intravitreal Injection Workflow
Background
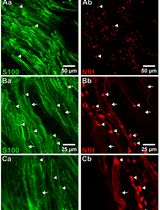
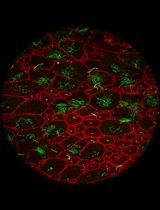
In the vertebrate visual system, retinal ganglion cells (RGCs) in the eye extend axons through the optic nerve to retinorecipient target areas in the brain that process visual information. Proteins perform many essential functions in cells, and the capacity to perform these functions depends on their subcellular distribution. Protein distribution in cells is tightly regulated, particularly in neurons, which are highly polarized with different cellular compartments in axons, dendrites, and cell bodies. A primary means of regulating protein distribution is by controlling their transport into axons and dendrites. To identify proteins that are transported from RGC somata into axons and synaptic targets in vivo, we labeled proteins in the retina in rodents using intravitreal injections of N-hydroxysuccinimidobiotin (NHS-biotin) and analyzed biotinylated proteins in optic nerves and their projections in the visual system using immunohistochemical, biochemical, and mass spectrometry methods. These studies identified thousands of proteins that are transported along RGC axons from the cell bodies in the retina to the presynaptic axon terminals in the target areas, including the lateral geniculate nucleus (LGN) and superior colliculus (SC) [1–3].
In vivo protein biotinylation with NHS-biotin has many advantages for the investigation of protein identification, distribution, and function: NHS-biotin is a membrane-permeable biotin reagent that binds covalently to exposed lysines and N-terminal amino acids of proteins, resulting in extensive protein labeling. Biotin is a naturally occurring molecule that binds to many proteins without altering their function or activities. NHS-biotin cannot be re-incorporated into other proteins after protein degradation because the succinimide group is quenched after reacting with amino groups and because the resulting biotin-tagged amino acids, such as biotinyl-lysine, cannot be charged onto tRNAs [4]. Injecting NHS-biotin in the relatively large intravitreal space in the eye, ~2 mm away from the neural retina, avoids damaging the retina or the optic nerve and allows NHS-biotin to be taken up by retinal cells that line the back of the eye, including RGC cell bodies. The intravitreal chamber serves as a natural reservoir for the injected NHS-biotin, increasing the opportunity for protein labeling. NHS-biotin-labeled proteins distribute throughout RGC cells, including axons and dendrites. The optic nerve exits the back of the eye and extends to the brain, so that proteins that are transported along the optic nerve to the LGN, SC, and other targets in the brain can be captured by isolating the optic nerves. Proteins that are transported to the retinorecipient targets can be identified and characterized by local dissection of the target areas [2]. We call this population of proteins the transported proteome, or the transportome. A subset of proteins that are transported from the RGC cell bodies to the LGN are further transported via geniculocortical axons to the visual cortex [1]. As additional uses, we applied this in vivo intravitreal protein labeling strategy to mice with mutations in motor proteins and demonstrated molecular mechanisms underlying protein transport [3]. Also, we used intravitreal injection of the noncanonical amino acid azidohomoalanine to label newly synthesized RGC proteins in a model of glaucoma-induced optic nerve injury, and identified changes in transport of newly synthesized proteins in response to optic nerve injury [5]. Additionally, we injected NHS-biotin unilaterally into one cortical lobe in mouse pups to label intercortical callosal axons [1]. Together, these studies represent a range of applications of intravitreal protein labeling strategies.
Here, we present a detailed protocol for intravitreal NHS-biotin injections, optic nerve dissection, and methods for minimally invasive and unbiased in vivo labeling of proteins and immunostaining of whole optic nerve samples to investigate protein transport along the entire optic nerve, enabling analysis of protein transport in the optic nerves of animals of different ages and genotypes.
Materials and reagents
Intravitreal injections
1. EZ-Link NHS-biotin (ThermoFisher, catalog number: 20217)
2. Hybri-Max DMSO (Sigma, catalog number: D2650)
3. 1.5 mL microfuge tubes (regular and light-blocking amber or cover with foil)
4. 0.22 μm centrifugal filters (MilliporeSigma, catalog number: UFC30GV0S)
5. 1 mL syringe [(BD, catalog number: 309659) or gel-loading tips (Eppendorf, catalog number: 930001007)]
6. Borosilicate glass capillaries (Sutter, catalog number: BF-150-110-10)
7. Ruler (metric)
8. Fine-tip permanent marker
9. Reverse-action/Self-closing forceps (Fine Science Tools, catalog number: 11487-11)
10. Whetstone or sandpaper (Fine Science Tools, catalog number: 29008-22)
11. Mineral oil
12. #55 forceps (Fine Science Tools, catalog number: 11255-30)
13. Absorbent underpads (VWR, catalog number: 56617-014)
14. Lab tape
15. Heating pads (2)
16. Anesthesia nose cone + tubing (Kent Scientific, catalog numbers: SOMNO-0801, SOMNO-0602)
17. Anesthesia induction chamber + tubing (Kent Scientific, catalog number: SOMNO-0710)
18. Ophthalmic ointment (Henry Schein, catalog number: 1371627)
19. Triple antibiotic ointment (Henry Schein, catalog number: 5703130)
20. Sterile cotton swabs
21. Isoflurane
22. VaporGuard gas scavengers (VetEquip, catalog number: 931401)
23. Mice (experiment-specific genotype; albino strains are ideal for learning/training)
24. Disposable nitrile gloves, lab coat, and additional PPE as needed
Dissection
Note: All dissections were performed as described by Cameron et al. [6]. Please refer to the referenced Bio-protocol article for a list of reagents and tools necessary to complete dissections.
1. 16% Paraformaldehyde ampules (Electron Microscopy Sciences, catalog number: 15710)
Immunolabeling
1. 16% Paraformaldehyde ampules (Electron Microscopy Sciences, catalog number: 15710)
2. Slides
3. 1.5 mL microfuge tubes
4. 48-well plate (can be reused)
5. Parafilm
6. No. 15 (or smaller) Feather scalpel (Electron Microscopy Sciences, catalog number: 7204215)
7. #1.5 Coverslips
8. Embryo/Ring forceps (Fine Science Tools, catalog number: 11103-09)
9. Pipetman + tips
10. Fine-tipped transfer pipettes (Samco, catalog number: 235)
11. Goat anti-biotin antibody (Thermo, catalog number: 31852)
12. Alexa-568 donkey-anti-goat antibody (Invitrogen, catalog number: A11057)
13. Hoechst 33342 (AnaSpec, catalog number: AS-83218)
14. FocusClear clearing solution (CelExplorer, catalog number: FC-101)
15. MountClear mounting media (CelExplorer, catalog number: MC-301)
Solutions
1. PTx wash buffer (see Recipes)
2. Permeabilization buffer (see Recipes)
3. Block-Perm buffer (see Recipes)
4. Block-antibody buffer (see Recipes)
Recipes
1. PTx wash buffer (1 L)
1× PBS pH 7.4 + 0.1% Triton X-100
2. Permeabilization buffer (50 mL)
1× PBS pH 7.4 + 0.5% Triton X-100
3. Block-Perm buffer (20 mL)
Permeabilization buffer + 4% bovine serum albumin + 1% donkey serum
4. Block-antibody buffer (20 mL)
PTx wash buffer + 4% bovine serum albumin + 1% donkey serum
Equipment
1. Bunsen burner
2. Sutter glass puller (Sutter Instruments, model: P-97), fitted with a box filament (FB330B)
3. Microfuge
4. Dissecting microscope with ample working distance and light source
5. PicoSpritzer (Parker, catalog number: 052-0500-900) (or Toohey Spritzer or other back-pressured injection system)
6. Micromanipulator (Narshige, model: MM-3)
7. Magnetic base (Narshige, model: GJ-8)
8. Iron plate (Narshige, IP)
9. Anesthesia machine/Hi-Flow Precision Vaporizer for isoflurane administration
10. Pelco BioWave Pro (Ted Pella, catalog number: 36700)
11. Orbital mixer/rotator (Light Labs, Mini LabRoller, catalog number: H5500)
Procedure
文章信息
稿件历史记录
提交日期: Mar 26, 2025
接收日期: Jul 9, 2025
在线发布日期: Jul 28, 2025
出版日期: Aug 20, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
McKeown, C. R., Schiapparelli, L. M. and Cline, H. T. (2025). Intravitreal NHS-Biotin Injection and Immunohistochemistry to Label and Image Protein Transport in the Mouse Optic Nerve. Bio-protocol 15(16): e5419. DOI: 10.21769/BioProtoc.5419.
分类
神经科学 > 神经解剖学和神经环路 > 视神经
神经科学 > 神经解剖学和神经环路 > 免疫荧光
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link