- EN - English
- CN - 中文
Integrated Membrane Yeast Two-Hybrid System for the Analysis of Membrane Protein Complexes
用于膜蛋白复合物分析的一体化膜型酵母双杂交系统
发布: 2025年08月20日第15卷第16期 DOI: 10.21769/BioProtoc.5418 浏览次数: 3014
评审: Asmita PawarAnonymous reviewer(s)

相关实验方案
![放射性 D-erythro-[4,5-³H] 二氢鞘氨醇标记酵母鞘脂的代谢方法](https://en-cdn.bio-protocol.org/imageup/arcimg/20130820012439267.jpg?t=1770095437)
放射性 D-erythro-[4,5-³H] 二氢鞘氨醇标记酵母鞘脂的代谢方法
Takefumi Karashima [...] Kouichi Funato
2013年08月20日 10502 阅读
Abstract
Protein–protein interactions facilitate cellular functions through the creation of networks and multi-protein complexes. Mapping the interactions within and between protein networks and elucidating the composition of protein complexes provides critical insight into biological processes. Interactions among soluble cytoplasmic proteins have been extensively investigated through the application of immunoaffinity capture as well as conventional nuclear two-hybrid testing. The integrated membrane yeast two-hybrid provides a method to investigate protein–protein interactions between integral membrane proteins in their native membrane environment. This procedure makes use of the ability of the amino-terminal fragment of ubiquitin (Nub) and the carboxyl-terminal fragment of ubiquitin (Cub) to refold reconstituting functional ubiquitin, which can be recognized by a ubiquitin peptidase. Appending a fusion protein composed of Cub fused to LexA and VP16 (CLV) to a candidate "bait" protein and Nub to candidate "prey" proteins allows a test of their interaction. If the two proteins interact closely, the CLV fragment is cleaved and enters the nucleus to activate the expression of reporter genes, signaling the interaction. When the bait and prey proteins are tagged with CLV and NubG, respectively, at their genomic loci, they are only copies of the bait and prey in the cell and are expressed under the regulation of their native promoters. This avoids overexpression artifacts that can occur if the tagged proteins are expressed from plasmids while the untagged chromosomally encoded copies of the bait and prey continue to be expressed.
Key features
• Allows an in vivo interaction test with integral membrane proteins in the native membrane environment.
• Allows integration of NubG tag at the amino or carboxyl-terminus of prey proteins.
• Avoids overexpression artifacts that can be caused by expression of CLV-tagged bait and NubG-tagged prey proteins from plasmid-based systems.
• Avoids competition from untagged chromosomally encoded bait and prey proteins, as occurs when CLV-tagged bait and NubG-tagged prey are expressed from plasmids.
Keywords: Yeast (酵母)Background
Protein–protein interactions drive and regulate a broad array of cellular processes [1]. Defining individual protein–protein interactions and mapping entire networks as interactomes has become a key tool for the interpretation of cellular function and complex phenotypes [2]. Protein species display tremendous diversity in structure and the chemical environment in which they accomplish their functions. This diversity ensures that no single approach is capable of effectively detecting all forms of protein–protein interactions that take place in any given cell type. In response to this challenge, a wide range of tools have been developed to investigate protein–protein interactions. These include biochemical strategies of co-fractionation, affinity purification, and proximity labeling [3,4]. Genetic approaches of two-hybrid and synthetic genetic arrays have also provided extensive data sets that add to the catalog of protein–protein interactions [5,6].
Any form of protein–protein interaction analysis that depends on protein isolation or purification has limitations regarding the ability to detect weak or transient interactions and the potential to generate false-positive interactions when cellular compartments are mixed following cell lysis. In vivo approaches to the analysis of protein–protein interactions benefit from sensitivity and an improved likelihood that proteins will retain their native structure. The nuclear yeast two-hybrid that detects the interaction between specified "bait" and "prey" proteins that are imported into the nucleus has allowed extensive interactome analysis and is effective for nuclear and many cytoplasmic proteins; however, some cannot interact correctly when removed from their native context and environment [7].
Integral membrane proteins that reside partially or entirely within the confines of a phospholipid bilayer compose around 30% of the proteome of characterized eukaryotic cells [8]. This class of proteins mediates, among other functions, communication, cell–cell interaction, transport of metabolites and small molecules, energy metabolism, and lipid biosynthesis. Considering the diverse functions and, in some cases, the cell surface localization, it is not surprising that membrane proteins are a large portion of drug targets [9]. The wide range of cellular processes controlled or influenced by this class of proteins highlights the importance of understanding their function and regulation. Proteins do not function in isolation, and their activity, localization, and modification state can be governed by their interaction with other proteins. Developing an understanding of these relationships among integral membrane proteins is challenging owing to the phospholipid membrane environment in which these proteins reside. The phospholipid membrane environment influences the structure and function of integral membrane proteins [10]. Investigations of the structure, function, and protein interactions of integral membrane proteins are complicated by this environment, as in many cases, removal from the membrane leads to loss of structural integrity and protein aggregation. Thus, not all interactions among integral membrane proteins can be reliably identified through biochemical methods that rely on protein removal from the native cellular environment.
A variety of techniques can be applied to investigating protein–protein interactions among integral membrane proteins, including affinity purification and co-immunoprecipitation of detergent-solubilized proteins [11,12]. These have limitations, namely the need to remove the proteins from their native lipid bilayer environment with potential for protein aggregation and loss of any low-affinity protein interactions. In vivo approaches, including proximity labeling experiments using fusions of a membrane protein to a biotin ligase, have been reported but have not been widely applied, possibly owing to the extensive analysis required to identify the binding partners [13]. A variety of fluorescence microscopy strategies, including fluorescence resonance energy transfer (FRET) and fluorescence recovery after photobleaching (FRAP), to investigate the interaction between integral membrane proteins, have been discussed in recent reviews [14,15]. These are dependent on high-resolution microscopy capabilities and require fusing the proteins of interest to fluorescent protein tags. They are most effective with larger cells, such as mammalian cells, and can be challenging when applied to small cells such as Saccharomyces cerevisiae.
A genetic approach based on the two-hybrid concept, modified for the detection of protein–protein interactions between integral membrane proteins, was pioneered by Dünnwald et al. [16]. This strategy was based on the small and highly conserved protein ubiquitin, which can be covalently ligated to target proteins by ubiquitin ligases and removed from proteins by ubiquitin peptidases (Ubp) [17]. A remarkable feature of ubiquitin is that the amino-terminal residues 1–34 can be expressed separately from the carboxyl-terminal residues 35–76; if the two halves are brought into close proximity, they will refold into a functional ubiquitin molecule that can be recognized by ubiquitin peptidases [18]. In this "split-ubiquitin" method, two proteins of interest can be fused: one with the amino-terminus of ubiquitin (Nub) and the other with the carboxyl-terminus of ubiquitin (Cub). If the two proteins interact with one another, the two fragments of ubiquitin will fold into a functional molecule [16]. The native yeast ubiquitin has an isoleucine residue at position 13 (Ile 13), providing the Nub fragment referred to as NubI with a very high affinity for Cub and the ability to spontaneously refold. The replacement of Ile 13 with a glycine residue creates NubG. This variant has a significantly reduced affinity for Cub and requires that NubG and Cub be in very close proximity before the two fragments can refold into a functional ubiquitin that can be recognized by ubiquitin peptidases [18]. Ubiquitin Ile 13 forms part of the hydrophobic core of the folded ubiquitin protein. The hydrophobic nature of this amino acid residue is important for the conformational stability of ubiquitin. Based on the crystal structure of ubiquitin, Ile13 is in a position to make hydrophobic interactions with amino acid Phe 3 and Val 5 [19]. A mutation of Ile 13 to glycine (Gly 13), creating NubG, reduces the potential for hydrophobic interactions at the amino-terminus of ubiquitin and reduces conformational stability of the protein molecule. The reduced conformational stability is reflected in a reduced ability to spontaneously refold, thus requiring the amino and carboxyl fragments of ubiquitin to be in very close proximity to allow refolding of Nub and Cub fragments and the reconstitution of Ubp cleavage [18].
A strategy utilizing a split-ubiquitin was directly applied to the analysis of membrane proteins by generating a reporter system that could detect when the two fragments of ubiquitin came into close contact and folded into a functional ubiquitin molecule [20]. This system, referred to as the membrane yeast two-hybrid (MYTH), makes use of a fusion protein composed of the Cub fragment fused to a DNA-binding protein domain from the E. coli LexA protein and a transcriptional activation domain from the Herpes simplex virus VP16 including a nuclear localization signal. This fusion protein Cub-LexA-VP16 is abbreviated to CLV. When the CLV fragment is fused to one protein (a bait protein), and the Nub fragment is fused to another (a prey protein), the fusions remain intact unless the bait and prey proteins bind to one another. If the two proteins bind, this brings CLV and NubG into close proximity, allowing the Nub and Cub fragments to fold and be recognized by cellular ubiquitin peptidases (Ubp). The Ubp can subsequently cleave the LexA-VP16 fragment from Cub of CLV, releasing LexA-VP16 to migrate into the nucleus (Figure 1). The detection mechanism for this event makes use of reporter genes under the regulation of upstream LexA operator sites (LexAop) so that when a LexA-VP16 fusion is released and enters the nucleus, it can bind the LexA operators, allowing the fused VP16 to trigger activation of the reporter genes (Figure 1). Common reporter genes used include HIS3, ADE2, and LacZ [20,21]. This genetic approach to testing for interactions between membrane proteins benefits from investigating the interactions in vivo, with the integral membrane proteins remaining in their native conformation and membrane environment. The approach has found wide application with systematic screens to characterize large interactomes among S. cerevisiae membrane proteins in the endoplasmic reticulum [22]. The strategy has also been applied to discover and characterize protein interactions and interactomes among mammalian and plant cell proteins [23–25].
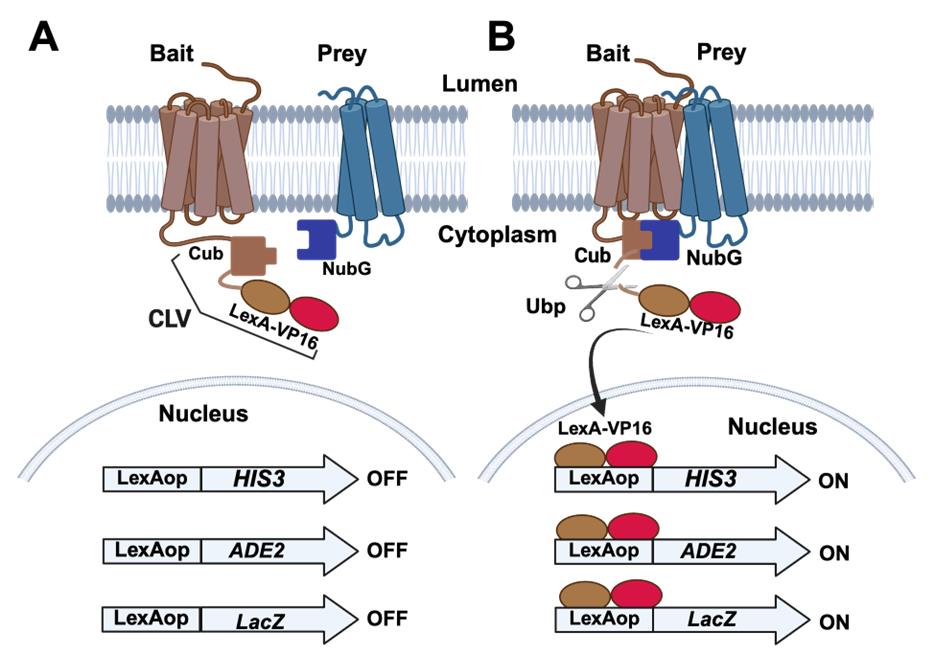
Figure 1. Split-ubiquitin membrane yeast two-hybrid (MYTH) systems. (A) The bait protein of interest is modified by the addition of a sequence coding for the carboxyl-terminus of ubiquitin (Cub) fused to the DNA binding protein LexA and transcriptional activation domain of VP16. This fusion is labeled as CLV in the figure. The prey protein is modified by the addition of a sequence coding for the amino-terminus of ubiquitin (NubG). The tester S. cerevisiae strain is also engineered by the addition of reporter genes under the regulation of LexA operator sites (LexAop) that can be bound by LexA. Commonly employed reporter genes include HIS3 and ADE2 that confer growth on medium lacking histidine and adenine. An additional reporter gene (LacZ) confers blue color development in the presence of X-Gal. In the absence of an interaction between the bait and prey proteins, the reporter genes are not activated, and yeast colonies will remain white in the presence of X-Gal and will not form in the absence of histidine and adenine. (B) In the case that the bait and prey proteins interact with one another, the Cub and NubG fragments will fold into a functional ubiquitin moiety and will be recognized for cleavage by ubiquitin peptidases (Ubp). This cleavage releases the LexA-VP16 fragment of the CLV fusion to migrate into the nucleus and activate the reporter genes, leading to blue colonies in the presence of X-Gal and growth on medium lacking histidine and adenine.
A variation of the MYTH assay that can be applied to investigations of S. cerevisiae membrane proteins is the integrated membrane yeast two-hybrid assay (iMYTH). In this approach, the endogenous gene encoding an integral membrane protein can be "tagged" with the CLV fusion to create a "bait" protein that can be used to identify interacting prey proteins [26].
Why use iMYTH?
The plasmid-borne split-ubiquitin approach has spawned a collection of plasmids that allow for the expression of amino and carboxyl-terminal fusion of CLV and Nub to bait and prey proteins [20]. These approaches are the only reasonable way to perform library screens where thousands of bait and prey proteins will be tested or when proteins from heterologous organisms are to be tested in S. cerevisiae. As a limitation, protein fusions will be overexpressed in the tester strain. When testing interactions between membrane proteins native to S. cerevisiae, if the CLV-tagged bait and NubG-tagged prey are placed under the regulation of their native promoters in episomal plasmids, both an untagged chromosomal gene and a tagged episomal gene will be expressed in the tester strain. This increases the expression of the bait and prey proteins and creates a situation where the tagged bait must compete with the untagged endogenous protein for binding partners. Additionally, overexpression can also induce mislocalization with the potential to induce false-positive interactions. Some integral membrane proteins are subject to regulation, which retains homeostasis over protein abundance. For example, the abundance of the essential ∆9 desaturase in S. cerevisiae, Ole1, is subject to regulation by the endoplasmic reticulum-associated degradation (ERAD) system; although Ole1 activity is essential, excess Ole1 is rapidly degraded [27]. When a plasmid-borne OLE1-CLV fusion is tested as a bait, this results in elevated auto-activation of the split-ubiquitin system, because ERAD-mediated degradation of the excess OLE1-CLV leads to release of the LexA-VP16 fusion and creates high background signal [28].
The iMYTH protocol is well-suited for testing interactions among protein species that naturally reside in the endoplasmic reticulum, peroxisome, or plasma membranes. The procedure can be effectively used for testing individual protein–protein interactions or for library screening with multiple bait proteins. One limitation of the iMYTH test for protein–protein interaction is that it cannot be employed if the CLV and/or NubG tags disrupt the structure or function of candidate bait and prey proteins. A related issue is that the topology of the bait and prey proteins must allow for both the CLV and NubG tags to be exposed on the cytosolic side of the membrane. Otherwise, they will not be accessible to the ubiquitin peptidases. When testing heterologous proteins not native to yeast in the iMYTH system, if the heterologous proteins require post-translational modifications for them to interact, those modifications may not occur in yeast, creating a limitation on the use of the assay. Similarly, heterologous proteins that interact with partners within the context of multiprotein complexes may not bind to specific partners in the absence of the entire complex. Additionally, heterologous proteins that display instability in S. cerevisiae may yield high background signals. Cases where the iMYTH assay is unsuitable for testing protein–protein interactions may be candidates for other procedures, including co-immunoprecipitation or microscopy-based co-localization studies. These alternative approaches have the advantage of being able to be performed on the cells where the proteins of interest naturally reside.
With the advent of PCR-mediated gene tagging [29], it has become possible to introduce fusions of the Cub-LexA-VP16 bait and NubG prey fusions into the chromosomal copies of the bait and prey genes. A primary advantage of employing an integrated bait or bait and prey approach is that the tagged proteins are retained under the regulation of their native promoters, and only one species of the protein is produced in the cells, rather than one tagged and one untagged version as would be the case if plasmid-borne copies of bait and/or prey were added to the cells. Thus, the tagged bait protein will be the only version participating in any complex formation in the cells. Further, this will aid in retaining stoichiometry within protein complexes and reduce the potential for false-positive signals that can be triggered by overexpression.
Materials and reagents
Biological materials
1. Escherichia coli DH5a F´/endA1 hsdR17 (rK– mK+) glnV44 thi-1 recA1 gyrA (NalR) relA1 Δ(lacIZYA-argF)U169 deoR (ϕ80dlacΔ(lacZ)M15) (E. coli Genetic Resource Center, CGSC:14231)
2. Saccharomyces cerevisiae strain NMY51 MATa, his3Δ200, trp1-901, leu2- 3,112, LYS2::4x(lexAop)-HIS3, ura3::8x(lexAop)-LacZ, ade2::8x(lexAop)-ADE2, GAL4 (LifeSciences Market, Hong Kong, catalog number: S0094)
Reagents
1. PageRulerTM prestained protein ladder (Thermo Fisher Scientific, catalog number: 26617)
2. SuperSignal West Pico Plus chemiluminescent substrate (Thermo Fisher Scientific, catalog number: 34577)
3. Antibody solutions
a. Primary: anti-HA HA.11 16B12 (1:10,000, mouse monoclonal antibody) (Covance, catalog number: 101R-500), anti-LexA (1:5,000, rabbit polyclonal) (EMD Millipore, catalog number: 06-719), anti-MYC, 9E10 (1:10,000, mouse monoclonal antibody) (Millipore Sigma, catalog number: M5546)
b. Secondary: anti-mouse HRP (1:5,000, goat anti-mouse IgG-HRP) (Bio-Rad, catalog number: 170-6516), anti-rabbit HRP (1:5,000, goat anti-rabbit IgG-HRP) (Bio-Rad, catalog number: 1706515)
4. Glycerol (Fisher Scientific, catalog number: 10795711)
5. Peptone (Fisher Scientific, catalog number: 211677)
6. Tryptone (BioShop Canada, catalog number: TRP402.5)
7. Glucose (Fisher Scientific, catalog number: 10141520)
8. Yeast extract (Fisher Scientific, catalog number: BP14222)
9. Lithium acetate dihydrate (Sigma-Aldrich, catalog number: L4158)
10. De-ionized water
11. Acetic acid (Fisher Scientific, catalog number: BP2401-500)
12. Salmon sperm DNA (ssDNA) (MilliporeSigma, catalog number: D1626)
13. Polyethylene glycol 3350 (BioShop Canada, catalog number: 335.1)
14. YNB without ammonium sulphate, without amino acids (BioShop Canada, catalog number: YNB404.1)
15. Potassium dihydrogen orthophosphate (Fisher Scientific, catalog number: 10783611)
16. Sodium phosphate dibasic (Na2HPO4) (Fisher Scientific, catalog number: BP332-500)
17. Sodium phosphate monobasic (NaH2PO4) (Fisher Scientific, catalog number: BP329-500)
18. Potassium acetate (KAc) (Fisher Scientific, catalog number: P181212)
19. Sodium acetate (NaOAc) (Fisher Scientific, catalog number: BP333-500)
20. Sodium carbonate (Na2CO3) (Fisher Scientific, catalog number: S263-500)
21. Potassium chloride (KCl) (Fisher Scientific, catalog number: BP366-1)
22. Dimethylsulfoxide (DMSO) (MilliporeSigma, catalog number: D8418)
23. Ethylenedinitrilotetraacetic acid (EDTA) (MilliporeSigma, catalog number: E6758)
24. Magnesium sulfate (MgSO4) (Fisher Scientific, catalog number: M65-500)
25. Trichloroacetic acid (TCA) (MilliporeSigma, catalog number: T9159)
26. Chloroform (Fisher Scientific, catalog number: C298-1)
27. Ethanol, 95%
28. 10 mM dNTP solution (Truin Science, catalog number: RTS5010)
29. Taq DNA polymerase (New England Biolabs, catalog number: M0267L)
30. Geneticin (G418) (Thermo Fisher Scientific, catalog number: 11811031)
31. Tris buffered phenol (Thermo Fisher Scientific, catalog number: 15513059)
32. RNase A (Fisher Scientific, catalog number: BP25391)
33. Ampicillin (Fisher Scientific, catalog number: BP1760-25)
34. Kanamycin sulfate (Thermo Fisher Scientific, catalog number: J17924.06)
35. Nourseothricin sulfate (MJS Biolynx, catalog number: JBAB10225G)
36. 5-bromo-4-chloro-3-indolyl-β-D-galactoside (X-Gal) (Fisher Scientific, catalog number: 15520034)
37. o-nitrophenyl-b-D-galactopyranoside (ONPG) (Thermo Fisher Scientific, catalog number: PI34055)
38. 0.5 mm acid-washed beads (Biospec products, catalog number: 11079105)
39. Agar (Fisher Scientific, catalog number: BP14232)
40. Agarose (Fisher Scientific, catalog number: BP1356500)
41. Adenine sulphate (MilliporeSigma, catalog number: A2545)
42. Uracil (Acros organics, catalog number: 157301000)
43. L-leucine (Fisher Scientific, catalog number: BP385-100)
44. L-tryptophan (MilliporeSigma, catalog number: T0254)
45. L-histidine (MilliporeSigma, catalog number: H8125)
46. L-methionine (Acros organics, catalog number: 166161000)
47. L-alanine (Fisher Scientific, catalog number: BP369-100)
48. L-arginine (Fisher Scientific, catalog number: BP370-100)
49. L-aspartic acid (Fisher Scientific, catalog number: BP374-100)
50. L-asparagine (Fisher Scientific, catalog number: BP373-100)
51. L-cysteine (MP Biomedicals, catalog number: 101444)
52. L-glutamic acid (MilliporeSigma, catalog number: 49449-100)
53. L-glutamine (MilliporeSigma, catalog number: 63126)
54. L-glycine (Fisher Scientific, catalog number: BP381-100)
55. L-isoleucine (Fisher Scientific, catalog number: BP384-100)
56. L-lysine (Fisher Scientific, catalog number: BP386-100)
57. L-phenylalanine (Fisher Scientific, catalog number: BP391-100)
58. L-proline (Fisher Scientific, catalog number: BP392-100)
59. L-serine (Fisher Scientific, catalog number: BP393-100)
60. L-threonine (Fisher Scientific, catalog number: BP394-100)
61. L-tyrosine (Fisher Scientific, catalog number: BP396-100)
62. L-valine (Fisher Scientific, catalog number: BP397-100)
63. 3-amino-1,2,4-trazole (3AT) (Millipore Sigma, catalog number: A8056)
64. Sodium dodecyl sulfate (SDS) (Bio-Rad, catalog number: 1610302)
65. DL-dithiothreitol (DTT) (Fisher Scientific, catalog number: BP172-5)
66. 30% Acrylamide 29:1 (Bio-Rad, catalog number: 1610156)
67. Bromophenol blue (Bio-Rad, catalog number: 161-0404)
68. Ammonium persulfate (Fisher Scientific, catalog number: 10020020)
69. TEMED (MilliporeSigma, catalog number: T9281)
70. Ammonium sulphate (Fisher Scientific, catalog number: BP212-212)
71. Tris base (Fisher Scientific, catalog number: BP152-1)
72. Glycine (Fisher Scientific, catalog number: 10070150)
73. Methanol (Fisher Scientific, catalog number: A412-4)
74. Sodium chloride (NaCl) (Fisher Scientific, catalog number: BP358-1)
75. Hydrochloric acid (HCl) (Fisher Scientific, catalog number: 351280-212)
76. Tween® 20 (Fisher Scientific, catalog number: BP151-500)
77. Gibson Assembly master mix (New England Biolabs, catalog number: E2621S)
78. Milk powder (Compliments, Canada)
79. Acetone (Fisher Scientific, catalog number: A18-1)
80. Triton X-100 (Fisher Scientific, catalog number: BP151-100)
Plasmid vectors
| Plasmid | Features | Marker | Source or reference |
|---|---|---|---|
| pUG-CLVt | Cub-LexA-VP16-KanR template | KanR | [28] |
| pADSL-Nx | High copy NubG prey plasmid C-term fusion | TRP1 | [42, 43] |
| pADSL-xN | High copy NubG prey plasmid N-term fusion | TRP1 | [42, 43] |
| pALG5-NubG | (negative control) prey plasmid | TRP1 | [42, 43] |
| pALG5-NubI | (positive control) prey plasmid | TRP1 | [42, 43] |
| pOST1-NubG | (negative control) prey plasmid | TRP1 | [42, 43] |
| pOST1-NubI | (positive control) prey plasmid | TRP1 | [42, 43] |
| pFUR4-NubG | (negative control) prey plasmid | TRP1 | [42, 43] |
| pFUR4-NubI | (positive control) prey plasmid | TRP1 | [42, 43] |
| pNAT-xN | Prey-NubG-NatR template | NatR | This work |
| pNAT-xNI | Prey-NubI-NatR template | NatR | This work |
| pNAT ADH-Nx | NatR-PADH1-NubG-prey template | NatR | This work |
Oligonucleotides
Oligonucleotides for iMYTH bait amplification
| Name | Sequence features 5′–3′ |
| F1 | (60 gene-specific nt)-G GCC ACT AGT ATG GAA CAA AAA C |
| R1 | (60 gene-specific nt)-TAC GCT GCA GGT CGA CAA CC |
| Integrated bait verification | |
| KanRscf | CCT CGA CAT CAT CTG CCC hybridize with KanR |
| ibvF | A 20-nucleotide primer corresponding to a sequence approximately 150–200 bp upstream of the stop codon of the gene of interest |
Open reading frame amplification for plasmid-borne prey
| Amino-terminal NubG fusions: | |
| Name | Sequence features 5′–3′ |
| NxF | GGT GGT CCA TAC CCA TAC GAT GTT CCA GAT TAC GCT GGA TCC-(gene-specific nt) |
| NxR | CTC GAG GTC GAC GGT ATC GAT AAG CTT GAT ATC GAA TTC T-(gene-specific nt) |
| Carboxyl-terminal NubG fusions: | |
| xNF | CAA TCA ACT CCA AGC TGG CCG CTC TAG AAC TAG TGG ATC C-(gene-specific nt) |
| xNR | AAC ATC GTA TGG GTA CAT ATC GAT AAG CTT GAT ATC GAA TTC-(gene-specific nt) |
Oligonucleotides for prey open reading frame amplification for integration
| Carboxyl-terminal NubG fusion | |
| Name | Sequence features 5′–3′ |
| NatxNF | (60 prey gene-specific nt)- TAC CCA TAC GAT GTT CCA GAT TAC |
| NatxNR | (60 prey gene-specific nt)- CGA CTC ACT ATA GGG AGA CC |
| Carboxyl-terminal NubG confirmation oligonucleotides: | |
| ipvCF | A 20-nucleotide primer corresponding to a sequence approximately 150–200 bp upstream of the final codon of the bait gene of interest |
| CYCt | CTT CCT TTT CGG TTA GAG CGG hybridize with CYCt. |
| Amino-terminal NubG fusion | |
| NatNxf | (60 prey gene-specific nt)-AAG CTT CGT ACG CTG CAG G |
| NatNxr | (60 gene-specific nt)-TTG ATA CCA CTG CTT GGA TCC |
| Amino-terminal NubG confirmation oligonucleotides: | |
| ADHnv | CTC GTC ATT GTT CTC GTT CCC hybridize with ADH1 promoter. |
| ipvNR | A 20-nucleotide primer corresponding to a sequence approximately 150–200 bp downstream of the start codon of the bait gene of interest |
Solutions
1. PCR reaction buffer (see Recipes)
2. 1 M Tris-HCl pH 8.0 (see Recipes)
3. 0.5 M EDTA (see Recipes)
4. 1 M LiAc (see Recipes)
5. 3 M sodium acetate pH 5.2 (see Recipes)
6. TE buffer (see Recipes)
7. TAE buffer (1 L 50× stock) (see Recipes)
8. Yeast lysis buffer (see Recipes)
9. 2× Sample buffer (see Recipes)
10. 12% Polyacrylamide separating gel (see Recipes)
11. 4% Polyacrylamide separating gel (see Recipes)
12. SDS running buffer (see Recipes)
13. TBST (see Recipes)
14. Blocking solution (see Recipes)
15. Semi-dry transfer buffer (see Recipes)
16. LiAc-TE (see Recipes)
17. 50% PEG (see Recipes)
18. 2 mg/mL salmon sperm DNA (see Recipes)
19. LiAc-PEG (see Recipes)
20. Z-buffer (see Recipes)
21. LB (see Recipes)
22. YEPD (see Recipes)
23. Synthetic dropout (SD) medium (see Recipes)
24. SD dropout mixture (see Recipes)
25. X-Gal (see Recipes)
26. Sodium phosphate solution (see Recipes)
27. X-Gal plates (see Recipes)
Recipes
1. PCR reaction buffer
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| 1 M Tris-HCl pH 8.8 | 20 mM | 20 μL |
| 1 M (NH4)2SO4 | 10 mM | 10 μL |
| 1 M KCl | 10 mM | 10 μL |
| 10% Triton X-100 | 0.1% | 10 μL |
| 1 M MgSO4 | 2 mM | 2 μL |
| H2O | n/a | 948 μL |
| Total | n/a | 1 mL |
2. 1 M Tris-HCl pH 8.0
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Tris base | 1 M | 121.1 g |
| H2O | n/a | to 1 L |
| Total | n/a | 1 L |
Dissolve the Tris base in 800 mL of water. Adjust the pH to 8.0 with concentrated HCl and make up the final volume to 1 L with H2O.
3. 0.5 M EDTA
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Na·EDTA·2H2O | 0.5 M | 181.6 g |
| H2O | n/a | to 1 mL |
| Total | n/a | 1 L |
Add the EDTA to about 800 mL of water and stir. Adjust pH to 8.0 with NaOH. The EDTA will not go into solution until the pH is near 8.0.
4. 1 M LiAc
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| CH3COOLi·2H2O | 1 M | 10.2 g |
| H2O | n/a | to 100 mL |
| Total | n/a | 100 mL |
5. 3 M sodium acetate pH 5.2
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Sodium acetate·3H2O | 3 M | 408.1 g |
| H2O | n/a | to 1 L |
| Total | n/a | 1 L |
Add the sodium acetate to about 800 mL of water and stir. Adjust pH to 5.2 with glacial acetic acid.
6. TE buffer
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| 1 M Tris-HCl pH 8.0 | 10 mM | 1 mL |
| 0.5 M EDTA pH 8.0 | 1 mM | 200 μL |
| H2O | n/a | to 100 mL |
| Total | n/a | 100 mL |
7. TAE buffer (1 L 50× stock)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Tris base | 2 M | 242 g |
| Glacial acetic acid | 1 M | 57 mL |
| 0.5 M EDTA pH 8.0 | 50 mM | 100 mL |
| H2O | n/a | to 1 L |
| Total | n/a | 1 L |
8. Yeast lysis buffer
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| 1 M Tris-HCl pH 7.5 | 50 mM | 0.5 mL |
| 0.5 M EDTA pH 8.0 | 20 mM | 0.4 mL |
| 10% SDS | 1% SDS | 1 mL |
| H2O | n/a | 8.1 mL |
| Total | n/a | 10 mL |
9. 2× Sample buffer
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| 1 M Tris-HCl pH 6.8 | 125 mM | 1.25 mL |
| 10% SDS | 4% SDS | 4 mL |
| Glycerol | 20% | 2 mL |
| Bromophenol blue | 0.01% | 0.001 g |
| H2O | n/a | 2.75 mL |
| Total | n/a | 10 mL |
10. 12% Polyacrylamide separating gel
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| ddH2O | n/a | 3.33 mL |
| 1.5 M Tris-HCl pH 8.8 | 375 mM | 2.5 mL |
| 30% acrylamide solution 29:1 | 12% | 4 mL |
| 10% SDS | 0.1% | 0.1 mL |
| Total | n/a | 10 mL |
11. 4% Polyacrylamide separating gel
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| ddH2O | n/a | 2.6 mL |
| 1 M Tris-HCl pH 6.8 | 126 mM | 0.63 mL |
| 30% acrylamide solution 29:1 | 4% | 0.67 mL |
| 10% SDS | 0.1% | 0.05 mL |
| Total | n/a | 5 mL |
12. SDS running buffer
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Glycine | 14.4 g/L | 14.4 g |
| Tris-base | 3.02 g/L | 3.02 g |
| SDS | 1 g/L | 1 g |
| H2O | n/a | to 1 L |
| Total | n/a | 1 L |
13. TBST
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Tris-base | 3 g/L | 3 g |
| NaCl | 8.0 g/L | 8 g |
| KCl | 0.2 g/L | 0.2 g |
| Tween-20 | 0.2% | 2 mL |
| H2O | n/a | to 1 L |
| Total | n/a | 1 L |
14. Blocking solution
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Skim milk powder | 5% | 5 g |
| TBST | n/a | 100 mL |
| Total | n/a | 100 mL |
15. Semi-dry transfer buffer
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Glycine | 39 mM | 1.9 g |
| Tris base | 48 mM | 2.5 g |
| 10% SDS | 0.037% | 3.7 mL |
| Methanol | 20% | 200 mL |
| H2O | n/a | 796.3 mL |
| Total | n/a | 1 L |
16. LiAc-TE
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| 1 M LiAc | 100 mM | 5 mL |
| TE buffer | n/a | 45 mL |
| Total | n/a | 50 mL |
17. 50% PEG
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| PEG 3350 | 50% | 50 g |
| H2O | n/a | to 100 mL |
| Total | n/a | 100 mL |
Add 50 g of PEG 3350 to about 30 mL of ddH2O. Gently warm the solution on a heated stir plate and stir until it is dissolved. Pour into a 100 mL measuring cylinder, bring the volume to 100 mL, and mix thoroughly. Transfer the solution to a glass storage bottle, and autoclave at 121 °C for 15 min. After autoclaving, add sterile water if needed to return the volume to 100 mL.
18. 2 mg/mL salmon sperm DNA
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Salmon sperm DNA | 50% | 200 mg |
| TE buffer | n/a | 100 mL |
| Total | n/a | 100 mL |
Prepare stocks by dissolving 200 mg of salmon sperm DNA in 100 mL of sterile TE by gently stirring using a stir bar and magnetic stir plate. This should take from a few hours to overnight. The dissolution can be accelerated using a wide-bore 25 mL pipette to pipette the mixture up and down until chunks of DNA are no longer visible. The DNA can be dispensed into aliquots of 1.0 mL in 1.5 mL microcentrifuge tubes and 5 mL samples in 15 mL sterile screw-capped plastic centrifuge tubes and stored at -20 °C. Immediately before use, denature the carrier DNA in a boiling water bath for 5 min and chill on ice.
19. LiAc-PEG
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| 50% PEG 3350 | 40% | 8 mL |
| 1 M LiAc | 100 mM | 1 mL |
| 2 mg/mL salmon sperm DNA | 0.3 mg/mL | 1.5 mL |
| 1 M Tris-HCl pH 8.0 | 10 mM | 0.1 mL |
| 0.5 M EDTA | 1 mM | 20 μL |
| Total | n/a | 10 mL |
20. Z-buffer
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Na2HPO4 | 60 mM | 16.1 g |
| NaH2PO4 | 40 mM | 5.5 g |
| KCl | 10 mM | 0.75 g |
| MgSO4 | 1 mM | 0.246 g |
| H2O | n/a | to 1 L |
| Total | n/a | 1 L |
21. LB
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| BactoTM tryptone | 10 g/L | 10 g |
| Yeast extract | 5 g/L | 5 g |
| NaCl | 5 g/L | 5 g |
| Agar | 20 g/L | 20 g |
| H2O | n/a | to 1 L |
| Total | n/a | 1 L |
Autoclave at 121 °C for 30 min. Pour plates to prepare solid medium.
22. YEPD
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| BactoTM peptone | 20 g/L | 20 g |
| Yeast extract | 10 g/L | 10 g |
| Glucose | 20 g/L | 20 g |
| Adenine sulfate | 40 mg/L | 40 mg |
| H2O | n/a | to 1 L |
| Total | n/a | 1 L |
Autoclave at 121 °C for 30 min.
23. Synthetic dropout (SD) medium
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Yeast nitrogen base | 1.6 g/L | 1.6 g |
| (NH4)2SO4 | 5 g/L | 5 g |
| Glucose | 20 g/L | 20 g |
| Amino acid dropout mixture | 2 g/L | 2 g |
| Agar | 20 g/L | 20 g |
| H2O | n/a | to 1 L |
| Total | n/a | 1 L |
Autoclave at 121 °C for 30 min. Pour plates to prepare solid medium. Note that the nomenclature for this form of medium indicates which nutrients are deficient. For example, medium lacking tryptophan and histidine would be indicated as SD -trp -his.
24. SD dropout mixture
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Adenine sulfate | 2.2% | 1 g |
| Leucine | 8.9% | 4 g |
| Uracil | 4.4% | 2 g |
| Alanine | 4.4% | 2 g |
| Arginine | 4.4% | 2 g |
| Aspartate | 4.4% | 2 g |
| Asparagine | 4.4% | 2 g |
| Cysteine | 4.4% | 2 g |
| Glutamate | 4.4% | 2 g |
| Glutamine | 4.4% | 2 g |
| Glycine | 4.4% | 2 g |
| Histidine | 4.4% | 2 g |
| Isoleucine | 4.4% | 2 g |
| Lysine | 4.4% | 2 g |
| Methionine | 4.4% | 2 g |
| Phenylalanine | 4.4% | 2 g |
| Proline | 4.4% | 2 g |
| Serine | 4.4% | 2 g |
| Threonine | 4.4% | 2 g |
| Tryptophan | 4.4% | 2 g |
| Tyrosine | 4.4% | 2 g |
| Valine | 4.4% | 2 g |
| Total | 45 g |
A specific dropout medium is prepared by excluding particular nutrients. For example, -trp -leu dropout is prepared as above, but without the addition of tryptophan and leucine.
25. X-Gal
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| X-Gal | 100 mg/mL | 0.1 g |
| Dimethylformamide | n/a | 1 mL |
| Total | n/a | 1 mL |
26. Sodium phosphate solution
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Na2HPO4 | 70 g/L | 70 g |
| NaH2PO4 | 30 g/L | 30 g |
| H2O | n/a | up to 1 L |
| Total | n/a | 1 L |
27. X-Gal plates
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Yeast nitrogen base | 1.6 g/L | 1.6 g |
| (NH4)2SO4 | 5 g/L | 5 g |
| Glucose | 20 g/L | 20 g |
| Amino acid dropout mixture | 2 g/L | 2 g |
| Agar | 20 g/L | 20 g |
| Sodium phosphate solution | 10% | 100 mL |
| 100 mg/mL X-Gal solution | 0.08 mg/mL | 0.8 mL |
| H2O | n/a | to 1 L |
| Total | n/a | 1 L |
Mix all components except for sodium phosphate and X-Gal. Autoclave at 121 °C for 30 min, cool to 50 °C, and then add the sodium phosphate and X-Gal. Mix briefly and then pour plates to prepare the solid medium. Store in the dark.
Laboratory supplies
1. Sterile toothpicks
2. Blue screw cap tubes, 50 mL (FroggaBio, catalog number: TB50-25)
3. Blue screw cap tubes, 15 mL (FroggaBio, catalog number: TB15-25)
4. Screw cap tubes, 2 mL (Sarstedt, catalog number: 72.693.005)
5. PCR tubes, 0.2 mL, domed cap (Axygen, catalog number: PCR-02D-C)
6. 1.5 mL microfuge tube (Fisher Scientific, catalog number: 05408129)
7. 25 mm Syringe filter 0.2 μm PES membrane (Fisher Scientific, catalog number: 13-1001-06)
8. Chromatography paper (Fisher Scientific, catalog number: 05-714-4)
9. Petri dishes, 100 mm (Fisher Scientific, catalog number: FB0875712)
10. Culture tubes 16 × 150 mm (MilliporeSigma, catalog number: CLS982016X)
11. Disposable cuvette (Fisher Scientific, catalog number: 14955127)
12. DNA Miniprep kit (Qiagen, catalog number: 27106)
13. 25-gauge needles (Fisher Scientific, catalog number: 1482649)
14. Immobilon-FL PVDF transfer membranes (Millipore, catalog number: IPFL00010)
Equipment
1. Spectrophotometer Ultraspec 3000 (Pharmacia-LKB, catalog number: 80-2106-25 DX)
2. Incubators (VWR, model: 1545, catalog number: 35823-204)
3. Water bath incubator (Thermo Fisher, catalog number: TSGP2S)
4. Microcentrifuge 5425R (Eppendorf Canada Ltd, catalog number: 5406000445)
5. Simpliamp thermal cycler (Thermo Fisher Scientific, catalog number: A24811)
6. Mini-protean vertical electrophoresis cell (Bio-Rad Laboratories, catalog number: 1658001FC)
7. Semi-dry transfer apparatus (Tyler Instruments, catalog number: TSD15-24)
8. Vortex (Fisher Scientific, catalog number: 12-812)
9. Imaging system, Gel-Doc XR (Bio-Rad Laboratories, catalog number: 170-8195)
10. Horizontal gel electrophoresis apparatus (Bio-Rad Laboratories, catalog number: 1704466)
11. Li-COR odyssey XF Western blot imaging system (LICORbio)
Procedure
文章信息
稿件历史记录
提交日期: May 2, 2025
接收日期: Jul 9, 2025
在线发布日期: Jul 27, 2025
出版日期: Aug 20, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Greenwood, B. L., David, K. O. A. and Stuart, D. T. (2025). Integrated Membrane Yeast Two-Hybrid System for the Analysis of Membrane Protein Complexes. Bio-protocol 15(16): e5418. DOI: 10.21769/BioProtoc.5418.
- Greenwood, B. L., Luo, Z., Ahmed, T., Huang, D. and Stuart, D. T. (2023). Saccharomyces cerevisiae Δ9-desaturase Ole1 forms a supercomplex with Slc1 and Dga1. J Biol Chem. 299(7): 104882. https://doi.org/10.1016/j.jbc.2023.104882
分类
微生物学 > 微生物蛋白质组学 > 膜蛋白
细胞生物学 > 细胞新陈代谢 > 脂质
系统生物学 > 相互作用组 > 蛋白质-配体相互作用
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link