- EN - English
- CN - 中文
A Simple and Adaptable Method for Cloning Genes Into Transposon Vectors Using Topo and Restriction Systems for Chicken Embryo Transgenesis
利用Topo与限制性系统将基因克隆至转座子载体的简便可适应方法及其在鸡胚转基因中的应用
发布: 2025年08月20日第15卷第16期 DOI: 10.21769/BioProtoc.5416 浏览次数: 1826
评审: Laxmi Narayan MishraAnonymous reviewer(s)

相关实验方案
一种快速且经济的流程,用于从细菌草图基因组中识别并捕获生物合成基因簇
Marco A. Campos-Magaña [...] Luis Garcia-Morales
2025年12月20日 760 阅读
Abstract
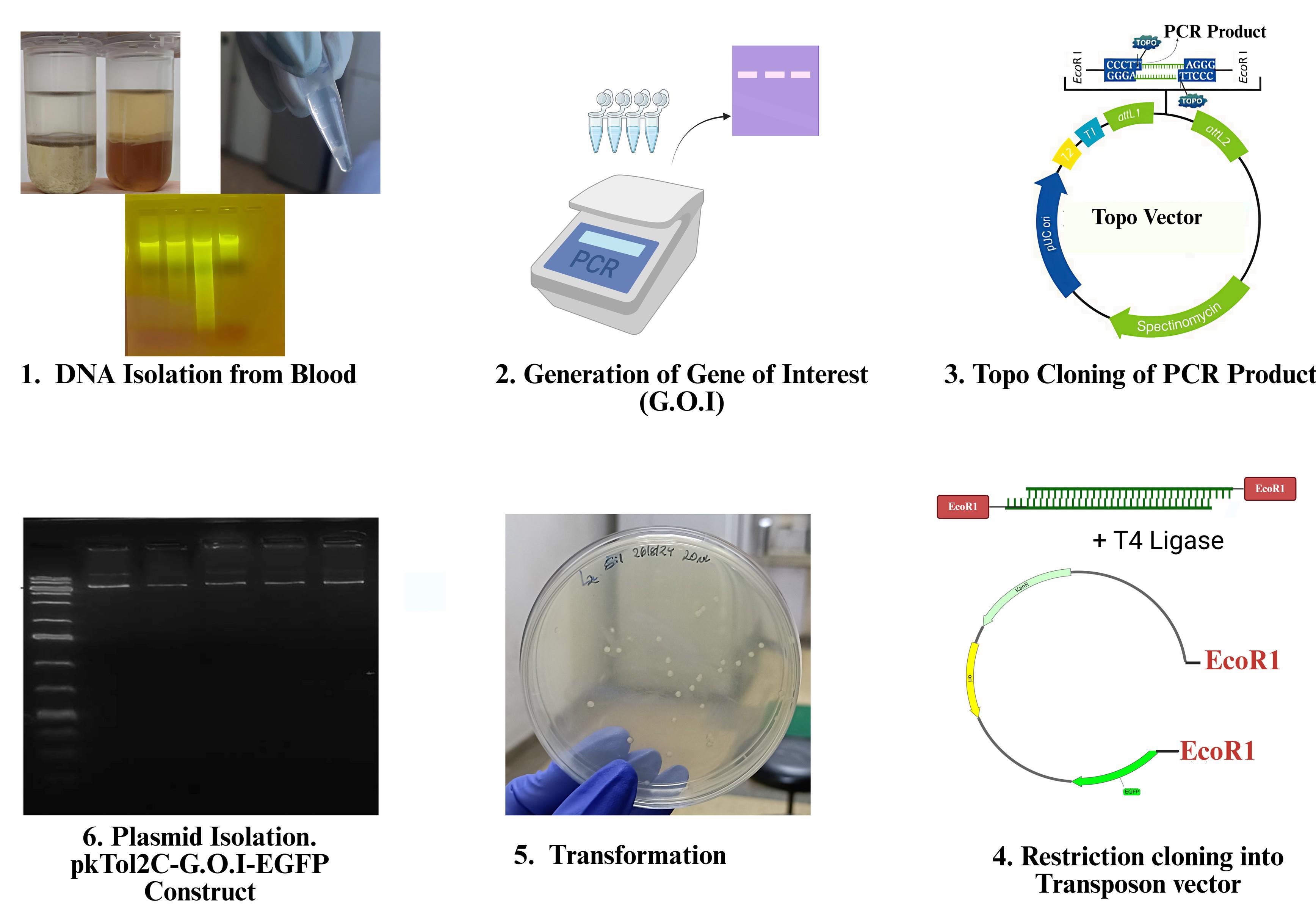
Transposon-based genetic transformation enables stable transgene integration in avian genomes and is increasingly used in the development of transgenic chickens for enhanced disease resistance, productivity, and biopharmaceutical applications. Conventional transformation techniques in avian biotechnology, including viral vectors and primordial germ cell (PGC) manipulation, are limited by biosafety risks, low efficiency, and technical complexity. This protocol outlines a two-step cloning approach for generating transposon-compatible gene constructs suitable for chicken embryo microinjection. Topoisomerase-based (TOPO) cloning is used as the first step due to its ability to directly clone PCR-amplified products without the need for restriction site-engineered primers while simultaneously producing an insert flanked with EcoRI restriction sites. The insert is subsequently transferred into the transposon vector through EcoRI-mediated restriction digestion and ligation. This approach simplifies construct generation by integrating the speed of TOPO cloning with the precision of restriction cloning, while ensuring compatibility with transposon-mediated integration systems. The protocol is efficient, reproducible, and does not require specialized equipment, providing a practical and scalable tool for gene construct assembly in avian transgenesis research.
Key features
• Uses TOPO PCR cloning for initial gene insertion.
• Applies restriction cloning to transfer inserts to the destination vector.
• Employs pKTol2C-EGFP as the final transposon vector.
• Suitable for generating constructs for chicken embryo transgenesis.
Keywords: Transgenesis (转基因)Graphical overview
Background
Genetic engineering in chickens has been used to create lines of chickens resistant to different poultry diseases and to introduce desired traits for agricultural or biomedical uses [1]. Both viral and non-viral techniques have been explored for generating genetically modified chickens. Historically, lentiviruses and retroviruses have been used as vectors to transfer genetic material into host cells [2,3]. The first successful production of transgenic chickens was achieved through injection of a retroviral vector carrying a transgene construct into chicken embryos at the blastodermal stage [4]. This strategy is useful but has drawbacks, such as insert size restrictions, transgene silencing, low germline transmission efficiency, and possible biosafety issues brought on by environmental viral shedding [5,6]. To address these drawbacks, non-viral transfection methods involving primordial germ cells (PGCs) have been developed. PGCs are isolated from embryonic tissues, cultured in vitro, genetically modified by transfection, and then reinjected into developing embryos or adult gonads [7]. However, there are drawbacks as well, like poor transfection efficiency, difficulties maintaining PGCs in long-term culture, and irregular germline transmission [8].
Transposon-based transformation has emerged as a potent tool for chicken transgenesis because it can mediate stable transgene expression without tissue-specific repression and integrate into the host genome efficiently [5]. It introduces genetic material into host cells by means of transposable elements, also known as "jumping genes." A transposase enzyme introduces and integrates a transposon containing the desired gene into the genome in this system [9,10]. Stable transgenic lines in chicken have been produced using this method with success [11–13]. Transposon systems have several advantages over viral and non-viral transformation methods, such as stable gene integration, lower biosafety risks, increased transgene carrying capacity, and compatibility with multiplexed gene delivery and insertional mutagenesis [14,15]. The procedure outlined here provides a methodical approach to creating gene expression cassettes that are suitable for transformation in chickens. It uses TOPO cloning as the initial cloning step to achieve high cloning efficiency and enable rapid insertion of PCR-amplified fragments into an entry vector, eliminating the need for restriction site-engineered primers and laborious ligation steps [16,17]. This technique makes use of DNA topoisomerase I, an enzyme that has dual roles as a ligase and a restriction enzyme [18]. Topo PCR cloning takes advantage of the single A-overhangs generated at the 3′ ends of PCR products when a non-proofreading DNA polymerase is used, allowing for direct ligation into the vector with complementary T-overhangs [19,20]. The procedure is simplified into three easy steps: mixing the PCR product with the TOPO vector, incubating at room temperature for a few minutes, and transforming competent E. coli cells [16]. This approach produces inserts flanked by EcoRI restriction sites, enabling restriction ligation of the gene of interest into the transposon vector. The transposon vector used in this protocol, pKTol2C-EGFP, employs a mini-CAG promoter to drive expression of the inserted gene, while an EGFP reporter gene allows for tracking of transgene integration within host cells [21, 22]. The vector is flanked by Tol2 transposon sequences, positioned immediately upstream of the promoter and downstream of the β-globin poly(A) signal termination sequence. These Tol2 sequences are recognized by the host-derived transposase, facilitating precise excision of the transgene and its stable integration into the host genome [22]. This hybrid strategy combines the speed and reliability of TOPO cloning with the flexibility of restriction-based assembly. This protocol offers a flexible approach for generating customized vectors suited to diverse applications, including gene expression, genome editing, and functional genomics. It supports the efficient assembly of constructs encoding disease resistance or productivity-enhancing traits, and is adaptable for use in chickens and other avian species relevant to research, biotechnology, and agriculture [13].
Materials and reagents
Biological materials
1. Blood from indigenous chickens (obtained from Embu and Makueni Counties)
2. One Shot Chemically Competent E. coli (Invitrogen, catalog number: K250020, preserved at -80 °C)
3. Plasmid pKTol2C-EGFP (Addgene, catalog number: 85598, preserved at -20 °C)
4. TOPO vector (Invitrogen, catalog number: K250020, preserved at -20 °C)
Reagents
1. UltraPure phenol:chloroform:isoamyl alcohol (Invitrogen, catalog number: 15593-049, stored at 4 °C)
2. SDS (Sigma-Aldrich, catalog number: 151-21-3)
3. Proteinase K (Promega, catalog number: V302B)
4. Chloroform (Sigma-Aldrich, catalog number: 67-66-3)
5. Isoamyl alcohol (Sigma-Aldrich, catalog number: 123-51-3)
6. Molecular-grade isopropanol (Scharlau, catalog number: 67-63-0)
7. Molecular-grade ethanol (Scharlau, catalog number: 64-17-5)
8. Nuclease-free water (Bioconcept, catalog number: 3-07F04-I)
9. SYBR Green I nucleic acid gel stain (MedChemExpress, catalog number: HY-K1004)
10. LE agarose powder (Cleaver Scientific, catalog number: 9012-36-6)
11. 5× TAE buffer (Glentham Life Sciences, catalog number: GB8437-1l)
12. 6× DNA loading dye (New England Biolabs, catalog number: B7025SVIAL)
13. O'GeneRuler 1 kb Plus DNA ladder (Thermo Scientific, catalog number: SM1343)
14. MyTaq DNA Polymerase (Bioline, catalog number: BIO-21105)
15. Oligonucleotides: Insert Forward Primer: 5'-AGACGAGTACACCGAGTATCCA-3', Insert Reverse Primer: 5'-GCACGGAGCTAACCTCACTT-3', Vector Forward Primer: 5'-GCAACGTGCTGGTTATTGTG-3'
16. Spectinomycin (Sigma-Aldrich, catalog number: 22189-32-8)
17. Kanamycin sulfate powder (Fisher Scientific, catalog number: BP 906-100)
18. LB agar Miller (Scharlau, catalog number: 01-385-500)
19. LB broth (Scharlau, catalog number: 02-384-500)
20. Calcium chloride (Oxford Labchem, catalog number: 10035-04-8)
Solutions
1. 0.1 M calcium chloride solution (see Recipes)
2. 1 M Tris-HCl stock solution pH 7.5 (see Recipes)
3. 0.5 M EDTA stock solution pH 8.0 (see Recipes)
4. 10% SDS stock solution (see Recipes)
5. 10 mg/mL proteinase K stock solution (see Recipes)
6. SDS Proteinase K Lysis Buffer (see Recipes)
7. 1× TAE buffer (see Recipes)
8. LB liquid media (see Recipes)
9. LB agar solid media (see Recipes)
10. Spectinomycin (see Recipes)
11. Kanamycin (see Recipes)
Recipes
1. 0.1 M calcium chloride solution
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Distilled water | n/a | 1 L |
| Calcium chloride | 0.1 M | 14.7 g |
2. 1 M Tris-HCl stock solution pH 7.5
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Distilled water | n/a | 500 mL |
| Tris-HCl | 1 M | 60.57 g |
3. 0.5 M EDTA Stock solution pH 8.0
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Distilled water | n/a | 500 mL |
| EDTA | 0.5 | 73.0625 g |
4. 10% SDS stock solution
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Distilled water | n/a | 500 mL |
| SDS powder | 10% | 50 g |
5. 10 mg/mL Proteinase K stock solution
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Nuclease-free water | n/a | 10 mL |
| Lyophilized proteinase K | 10 mg/mL | 100 mg |
6. SDS proteinase K lysis buffer
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| 1 M Tris-HCl stock solution | 0.1 M | 10 mL |
| 0.5 M EDTA stock solution | 0.05 M | 10 mL |
| 10% SDS stock solution | 1% | 10 mL |
| 10 mg/mL Proteinase K | 100 µg/mL | 1 mL |
| Distilled water | n/a | 69 mL |
7. 1× TAE buffer
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Distilled water | n/a | 980 mL |
| 5× TAE | 1× | 20 mL |
8. LB liquid media, pH 7.0
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Distilled water | n/a | 1 L |
| LB broth powder | 25 g/L | 25 g |
9. LB agar media, pH 7.0
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Distilled water | n/a | 1 L |
| LB agar | 40 g/L | 40 g |
10. Spectinomycin
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Distilled water | n/a | 10 mL |
| Spectinomycin | 100 mg/mL | 1 g |
11. Kanamycin
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Distilled water | n/a | 20 mL |
| Kanamycin | 50 mg/mL | 1 g |
Laboratory supplies
1. Petri dishes (BoenMed, catalog number: 620201)
2. Corning PYREX round media storage bottles (Fisher Scientific, catalog number: 10462716)
3. Powder-free nitrile gloves (Medisolve, catalog number: E2022-06-01)
4. Parafilm (Amcor, catalog number: PM996)
5. PCR reaction strips (Genaxy Scientific, catalog number: GEN-0108-C-PCR)
6. 10 μL pipette tips (Genaxy Scientific, catalog number: GEN-UT-10-C)
7. 200 μL pipette tips (Genaxy Scientific, catalog number: GEN-UTG-200-Y)
8. 1,000 μL pipette tips (Genaxy Scientific, catalog number: GEN-UT-1000-B)
9. P10 micropipette (Eppendorf, catalog number: 4861000-0005)
10. P1000 micropipette (Eppendorf, catalog number: 4861000-0001)
11. P200 micropipette (Eppendorf, catalog number: 3123000055)
12. 50 mL Falcon tubes (Thermo Fisher Scientific, catalog number: AM12502)
13. 1.5 mL microcentrifuge safe-lock tubes (Genaxy Scientific, catalog number: GEN-MT-150-C)
14. Cell spreaders (Chemglass Life Sciences, catalog number: CLS-1350-02)
15. Spectrophotometer cuvettes (Thermo Fisher Scientific, catalog number: 14-955-127)
16. Syringe microfilters 0.22 μm (Sigma-Aldrich, catalog number: SLGVR33RS)
17. GeneJET Plasmid MiniPrep kit (Thermo Fisher, catalog number: K0502)
18. pCR 8/GW/TOPO TA Cloning kit (Invitrogen, catalog number: K250020)
19. ISOLATE II PCR and Gel kit (Bioline, catalog number: BIO52059)
20. Zymoclean Gel DNA Recovery kit (Zymo Research, catalog number: D4007)
21. EcoRI Enzyme (New England Biolabs, catalog number: R0101S)
22. T4 DNA ligase (New England Biolabs, catalog number: M0202S)
23. Shrimp alkaline phosphatase (New England Biolabs, catalog number: M0371S)
24. O'GeneRuler 1 kb Plus DNA ladder (Thermo Scientific, catalog number: SM1343)
Equipment
1. Nanodrop (Thermo Fisher Scientific, Model: Nanodrop One C)
2. Thermocycler (Cleaver Scientific, catalog number: GTC96S-230)
3. Weighing scale (Adam Equipment, serial number: AEAAY68)
4. -20 °C freezer (Haier Biomedical, model: DW-25L92)
5. Refrigerator 4–8 °C (Haier Biomedical, model: HLR-310F)
6. Gel Doc (UVITEC Cambridge, model: UVIDOC-HD6T, TOUCH-PLUS)
7. Biosafety cabinet (Haier Biomedical, model: HR1200-IIA2-S)
8. Gel electrophoresis system (Cleaver Scientific, model: NANOPAC-300P)
9. Autoclave (Tuttnauer, model: T-LAB ECO V85)
10. Water bath (Cole-Parmer, model: BWS-8/78905-21)
11. Refrigerated microcentrifuge (Biobase, model: BKC-PCR16)
12. Microcentrifuge (Eppendorf, serial number: 5427LO534084)
13. Incubator with shaker option (Major Science, model: SI-200)
14. Spectrophotometer (Eppendorf, model: 6135)
15. Vortex (Thermo Fisher Scientific, catalog number: 9501200)
Software and datasets
1. NEBioCalculator v1.15.7 (20/8/2024)
Procedure
文章信息
稿件历史记录
提交日期: May 16, 2025
接收日期: Jul 11, 2025
在线发布日期: Jul 24, 2025
出版日期: Aug 20, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Kirimi, P., Okumu, N., Maingi, J. M., Ngeranwa, J., Nyaga, P. and Binepal, Y. (2025). A Simple and Adaptable Method for Cloning Genes Into Transposon Vectors Using Topo and Restriction Systems for Chicken Embryo Transgenesis. Bio-protocol 15(16): e5416. DOI: 10.21769/BioProtoc.5416.
分类
分子生物学 > DNA > DNA 克隆
微生物学 > 异源表达系统
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link