- EN - English
- CN - 中文
Establishing and Maintaining 3D Organoid Cultures From Human Fallopian Tube Epithelium
来源于人输卵管上皮的三维类器官培养的建立与维持
发布: 2025年08月20日第15卷第16期 DOI: 10.21769/BioProtoc.5412 浏览次数: 2195
评审: Anu ThomasAnonymous reviewer(s)
Abstract
The female reproductive tract is comprised of different regions, each with distinctive physiological characteristics. One of them is the fallopian tubes, which are vital for human reproductive health and success. The ability to model their function and physiology is of utmost importance. So far, in vitro models have been based on a few immortalized or cancer cell lines derived from fallopian tube cells that lacked differentiated, specialized cell types and did not allow for the study of cancer initiation due to their implicit biases. Organoids, in contrast, overcome these limitations and provide an advanced, three-dimensional system for the study of healthy fallopian tube physiology and pathology. Fallopian tube organoids are comprised of epithelial progenitors that can be enriched using chemical or hormonal treatment into the different cell types that are found in the in vivo tissue, namely detyrosinated-tubulin-positive ciliated cells or paired-box protein 8 (PAX8)-positive secretory cells. This protocol provides a step-by-step guide for the establishment and maintenance of a long-term culture of organoids from healthy human fallopian tube tissue. The organoid model described here closely mimics the in vivo physiology and anatomy of human fallopian tube epithelium and provides a comprehensive basis for future studies on its underlying molecular characteristics and possible pathology.
Key features
• Provides a step-by-step guide for the establishment of long-term fallopian tube organoid cultures.
• Allows for rapid extension of fallopian tube epithelial progenitor cells with a yield of up to 1 × 108 cells within 3 weeks of isolation.
• Fallopian tube organoids closely mimic healthy physiology, being comprised of multiple different cell types, like detyrosinated-tubulin-positive ciliated cells or paired-box protein 8 (PAX8)-positive secretory cells.
• Further enrichment of secretory cells by hormonal treatment and ciliated cells by chemical treatment is possible.
Keywords: Organoids (类器官)Graphical overview
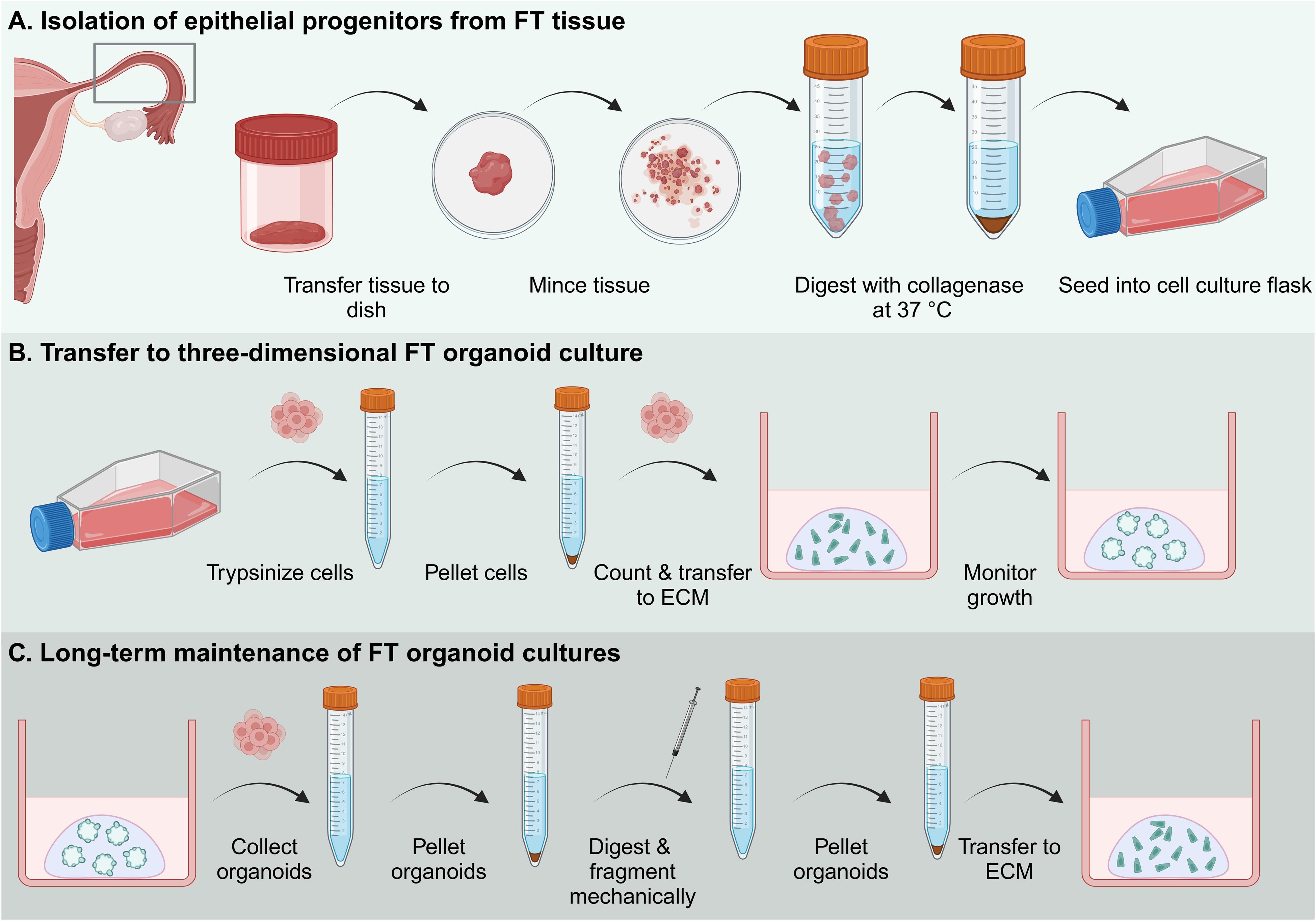
Graphical overview of the establishment and maintenance of human fallopian tube organoid cultures. Abbreviations: FT: fallopian tube; ECM: extracellular matrix.
Background
Since the initial description by Sato et al. [1], organoid technology has been expanded across entities and species barriers. In the past, researchers have predominantly relied on immortalized cancer cell lines or mouse models to study cancer biology, and both have their distinct advantages and shortcomings [2,3]. In comparison to these models, organoids have the advantage of primary origin and multiple differentiated cell types in combination with comparable low costs, scalability, and three-dimensional architecture [2,4]. At the same time, they retain patient characteristics, making them a powerful tool for disease modeling and cancer research as so-called patient avatars [4–6], personalized models derived from patient material representing individual patient characteristics and genotype, allowing for targeted therapeutic recommendations [4]. Previous work has focused on the establishment of organoids derived from healthy tissue from human intestines [7], liver [8], pancreas [9], stomach [10], and other organs. Here, we present a protocol for the establishment of organoids from a vital organoid for reproductive success, the fallopian tubes, based on our earlier descriptions [11]. The fallopian tubes are tube-like organs of the female reproductive system. Their primary function is to transport oocytes from the adjacent ovaries for fertilization and subsequently the embryo to the uterus for implantation [12]. For this, the tubes are equipped with an epithelium composed of secretory and ciliated cells that undergo dynamic change within the reproductive and hormonal cycle [13].
We previously showed that the establishment of proliferative cultures of fallopian tube epithelial progenitors is dependent on active Notch and WNT-signaling and requires constant supplementation of adequate ligands in the growth media [11]. These organoids closely mimic the in vivo differentiation [11] by differentiation of epithelial progenitors into detyrosinated-tubulin-positive ciliated cells and PAX8-positive secretory cells that can be further enriched by supplementing with chemical or hormonal treatments, such as dibenzazepine or β-estradiol and progesterone, respectively. The model proposed here has been successfully applied in the study of fallopian tube physiology [11], infection [14], and cancer initiation [15,16] by us and others.
Materials and reagents
Note: Examples for providers/manufacturers are given; similar cells, reagents, supplies, and devices might perform equivalently but have not been tested for these procedures.
Biological materials
1. L-Wnt3a-producing cell line (ATCC, catalog number: CRL-2647 [17], RRID: CVCL_0635)
2. R-Spondin-1-producing cell line (i.a. Cultrex HA-R-Spondin1-Fc 293T Cells, catalog number: 3710-001-01 or Kim, et al. [18])
Critical: Gene technological approval from the local authority must be obtained when working with GMOs.
Note: The conditioned media from the L-Wnt3a, R-Spondin-1, and Noggin can be replaced by the addition of 25% conditioned media of the L-WRN cell line (ATCC, catalog number: CRL-3276 [19], RRID: CVCL_DA06). A protocol for the generation of the conditioned media can be found at ATCC or in Warschkau et al. [20].
Reagents
1. L-Wnt3a conditioned medium [17] (made in-house; aliquot and store at -80 °C)
2. R-Spondin-1-conditioned medium [18] (made in-house; aliquot and store at -80 °C)
3. Extracellular matrix; growth factor reduced, i.e., Matrigel (Corning, catalog number: 354230; aliquot and store at -80 °C) or Cultrex BME type 2 (R&D Systems, catalog number: 3533-010-02; aliquot and store at -80 °C)
Note: The protocols listed here were established using Matrigel and Cultrex. The use of another substitute may affect organoid growth.
4. Fetal calf serum (FCS) (Corning, catalog number: A5256702; aliquot and store at -20 °C)
5. Advanced DMEM/F12 (Gibco, catalog number: 12634010; store at 4 °C)
6. B27 (Thermo, catalog number: 17504044; store at -80 °C) or alternatively NCS21 (Capricorn, catalog number: C21-H; store at -80 °C)
7. N2 for organoid medium (Capricorn, catalog number: N2-K; store at -80 °C)
8. Recombinant human epithelial growth factor (Thermo, catalog number: AF-100-15; dissolve in Advanced DMEM/F12, aliquot, and store at -80 °C)
9. Human recombinant Noggin (Thermo, catalog number: 120-10C; dissolve in DPBS containing 0.1% bovine serum albumin, aliquot, and store at -80 °C)
Note: The recombinant Noggin can be replaced by Noggin-secreting cell lines when the conditioned medium is added in a concentration of 10% (v/v).
10. Human recombinant fibroblast growth factor 10 (FGF10) (Thermo, catalog number: 100-26; dissolve in DPBS containing 0.1% bovine serum albumin, aliquot, and store at -80 °C)
11. Nicotinamide (Sigma, catalog number: N0636; dissolve in Advanced DMEM/F12, aliquot, and store at -80 °C)
12. N-acetyl-L-Cysteine (Sigma, catalog number: A9165; dissolve in Advanced DMEM/F12, aliquot, and store at -80 °C)
13. A83-01 (Biomol, catalog number: Cay9001799; dissolve in Advanced DMEM/F12, aliquot, and store at -80 °C)
14. Y-27632 (Biomol, catalog number: Cay9001799; dissolve in Advanced DMEM/F12, aliquot, and store at -80 °C)
15. HEPES (PanBioTech, catalog number: P05-01100; store at 4 °C)
16. GlutaMax (Gibco, catalog number: 35050038; store at 4 °C)
17. CryoSFM (PromoCell, catalog number: C-29922; store at 4 °C)
18. DPBS (PanBioTech, catalog number: P04-36500; store at 4 °C)
19. TrypLE Express (Gibco, catalog number: 12605010; store at 4 °C)
20. Penicillin/Streptomycin (Gibco, catalog number: 15140122; aliquot and store at -20 °C)
21. Fungin (InvivoGen, catalog number: ant-fn-1; store at -20 °C)
22. Gentamicin (Thermo, catalog number: 15750060; aliquot and store at -20 °C)
23. Collagenase type I (Sigma-Aldrich, catalog number: SCR103, dissolve in ethanol, aliquot, and store at -80 °C)
24. Dibenzazepine (DBZ, YO-01027) (MedChemExpress, catalog number: HY-13526; dissolve in DMSO, aliquot, and store at -80 °C)
25. β-estradiol (Sigma-Aldrich, catalog number: E2257; dissolve in ethanol, aliquot, and store at -80 °C)
26. Progesterone (Sigma-Aldrich, catalog number: P8783; dissolve in ethanol, aliquot, and store at -80 °C)
27. Dimethyl sulfoxide (DMSO) (Sigma-Aldrich, catalog number: D2650; store at room temperature)
28. Bovine serum albumin (BSA) (Carl Roth, catalog number: 3854; store at 4 °C)
Solution
1. ADF+++ (see Recipes)
2. FT isolation medium (see Recipes)
3. FT expansion medium (see Recipes)
Recipes
1. ADF+++
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Advanced DMEM/F12 | - | 500 mL |
| HEPES | 10 mM | 5 mL |
| GlutaMax | 1× | 5 mL |
| Penicillin/Streptomycin | 1% (v/v) | 5 mL |
2. FT isolation medium
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Human recombinant epithelial growth factor (EGF) | 10 ng/mL | 100 μL of a 10 µg/mL stock |
| Fetal calf serum | 5% (v/v) | 5 mL |
| Y-27632 (ROCK inhibitor) | 10 μm | 100 μL of a 10 mM stock |
| HEPES | 10 mM | 1,000 μL of a 1 M stock |
| GlutaMax | 1× | 1,000 μL |
| Penicillin/Streptomycin | 1% (v/v) | 1,000 μL |
| Fungin (only for isolation) | 0.1% (v/v) | 100 μL |
| Gentamicin (only for isolation) | 10 µg/mL | 100 μL of a 10 mg/mL stock |
| Advanced DMEM/F12 | - | Top up to 100 mL |
Note: Filter the freshly made medium through a 0.22 μm filter to avoid contamination and keep at 4 °C for a maximum of two weeks.
3. FT expansion medium
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Wnt3a conditioned medium [17] | 25% (v/v) | 25 mL |
| Rspondin-1 conditioned medium [18] | 25% (v/v) | 25 mL |
| B27 or NCS21 supplement | 2% (v/v) | 2 mL |
| N2 supplement | 1% (v/v) | 1 mL |
| Human recombinant epithelial growth factor (EGF) | 10 ng/mL | 100 μL of a 10 µg/mL stock |
| Human recombinant Noggin | 100 ng/mL | 100 μL of a 100 µg/mL stock |
| Human recombinant fibroblast growth factor 10 (FGF10) | 100 ng/mL | 100 μL of a 100 µg/mL stock |
| Nicotinamide | 1 mM | 100 μL of a 1 M stock |
| A83-01 (TGF-β inhibitor) | 1 μm | 100 μL of a 1 mM stock |
| Y-27632 (ROCK inhibitor) | 10 μm | 100 μL of a 10 mM stock |
| HEPES | 10 mM | 1,000 μL of a 1 M stock |
| GlutaMax | 1× | 1,000 μL |
| Penicillin/Streptomycin | 1% (v/v) | 1,000 μL |
| Advanced DMEM/F12 | - | Top up to 100 mL |
Note: Filter the freshly made medium through a 0.22 μm filter to avoid contamination and keep at 4 °C for a maximum of one week. The conditioned media from the L-Wnt3a, R-Spondin-1, and the recombinant Noggin can be replaced by the addition of 25% conditioned media of the L-WRN cell line (ATCC, CRL-3276 [19]). The recombinant Noggin can be replaced by Noggin-secreting cell lines when the conditioned medium is added in a concentration of 10% (v/v).
Laboratory supplies
1. 24-well tissue culture plates (F-base; TPP, catalog number: 920 12/24; or comparable product)
2. T25/T75 flasks (Sarstedt, catalog numbers: 83.3910.302/83.3911.302; or comparable product)
3. 15/50 mL conical centrifuge tubes (TPP, catalog number: 910 15/50; or comparable product)
4. 10/200/1,000 μL ART barrier pipette tips (Thermo Scientific, catalog numbers: 10098960/10029040/10313272; or comparable product)
5. Vacuum filtration system "rapid"-Filtermax (0.22 μm) (TPP, catalog number: 99505; or comparable product)
6. Sartorius MinisartR sterile filters (0.22 μm) (Thermo Scientific, catalog number: 10730792; or comparable product)
7. Duran glass beakers (25/600 mL) (Schott, catalog numbers: 2110614, 2110648; or comparable product)
8. Duran glass bottles (500/1,000 mL) (Schott, catalog numbers: 4459/5455; or comparable product)
9. B Braun solo cone Luer syringes (1 mL/20 mL) (Braun, catalog number: 12752637; or comparable product)
10. Single-use 18 G syringe needles (blunt, 40 mm) (VWR, BD Medical, catalog number: BDAM303129)
11. Disposable, sterile scalpels (Type 11 or 22; Feather; or comparable product)
12. Disposable, sterile forceps (Faust, MediSet, catalog number: 7661698; or comparable product)
13. Cell strainer (100 μm pore size) (PluriSelect, catalog number: 43-50100-51; or comparable product)
14. CoolCell LX cell freezing vial containers (Fisher Scientific, Corning, catalog number: 15572771; or comparable product)
15. Cell culture dishes (100 × 22 mm) (Th. Geyer, LabSolute, catalog number: 7696774; or comparable product)
Equipment
1. Safety cabinet for BSL-2 work, Safe 2020 Class II biological safety cabinet (Thermo Scientific, catalog number: 51026640; or comparable device)
2. Shaking water bath (Lauda, catalog number/model: Hydro H 20 S; or comparable device)
3. Centrifuge for 50/15 mL tubes (Hettich, catalog number/model: Rotina 420R; or comparable device)
4. Incubator (Binder, catalog number/model: CB-UL; or comparable device)
5. Rocker (IKA, KS 4000 i Control; or comparable device)
6. Automated cell counter (DeNovix, CellDrop BF; or comparable device)
7. Disposable hemocytometer (Kisker Biotech, catalog number: M-NZ; or comparable product) as an alternative for manual cell counting
8. Standard benchtop brightfield microscope for cell monitoring
Procedure
文章信息
稿件历史记录
提交日期: May 5, 2025
接收日期: Jul 9, 2025
在线发布日期: Jul 24, 2025
出版日期: Aug 20, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Holthaus, D., Hedemann, N., Geweniger, S. T., Le, H. D., Alkatout, I., Kessler, M. and Meyer, T. F. (2025). Establishing and Maintaining 3D Organoid Cultures From Human Fallopian Tube Epithelium. Bio-protocol 15(16): e5412. DOI: 10.21769/BioProtoc.5412.
分类
细胞生物学 > 细胞分离和培养 > 3D细胞培养
发育生物学 > 形态建成 > 器官形成
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link