- EN - English
- CN - 中文
Luminal Cerebrovascular Proteomics
脑血管腔面蛋白质组学研究
发布: 2025年08月05日第15卷第15期 DOI: 10.21769/BioProtoc.5411 浏览次数: 2572
评审: Pilar Villacampa AlcubierreAnonymous reviewer(s)
Abstract
Brain endothelial cells, which constitute the cerebrovasculature, form the first interface between the blood and brain and play essential roles in maintaining central nervous system (CNS) homeostasis. These cells exhibit strong apicobasal polarity, with distinct luminal and abluminal membrane compositions that crucially mediate compartmentalized functions of the vasculature. Existing transcriptomic and proteomic profiling techniques often lack the spatial resolution to discriminate between these membrane compartments, limiting insights into their distinct molecular compositions and functions. To overcome these limitations, we developed an in vivo proteomic strategy to selectively label and enrich luminal cerebrovascular proteins. In this approach, we perfuse a membrane-impermeable biotinylation reagent into the vasculature to covalently tag cell surface proteins exposed on the luminal side. This is followed by microvessel isolation and streptavidin-based enrichment of biotinylated proteins for downstream mass spectrometry analysis. Using this method, we robustly identified over 1,000 luminally localized proteins via standard liquid chromatography–tandem mass spectrometry (LC–MS/MS) techniques, achieving substantially improved enrichment of canonical luminal markers compared with conventional vascular proteomic approaches. Our method enables the generation of a high-confidence, compartment-resolved atlas of the luminal cerebrovascular proteome and offers a scalable platform for investigating endothelial surface biology in both healthy and disease contexts.
Key features
• Enables high-resolution proteomic profiling of the luminal surface of the brain vasculature in vivo.
• Improves signal-to-noise ratio through an added microvessel isolation step, reducing nonspecific background.
• Applied to uncover aging-related changes in luminal endothelial surface protein composition.
• Adaptable for identifying therapeutic targets, transporters, signaling pathways, and disease-associated alterations in the luminal vascular environment across diverse biological contexts.
Keywords: Vasculature (血管系统)Graphical overview
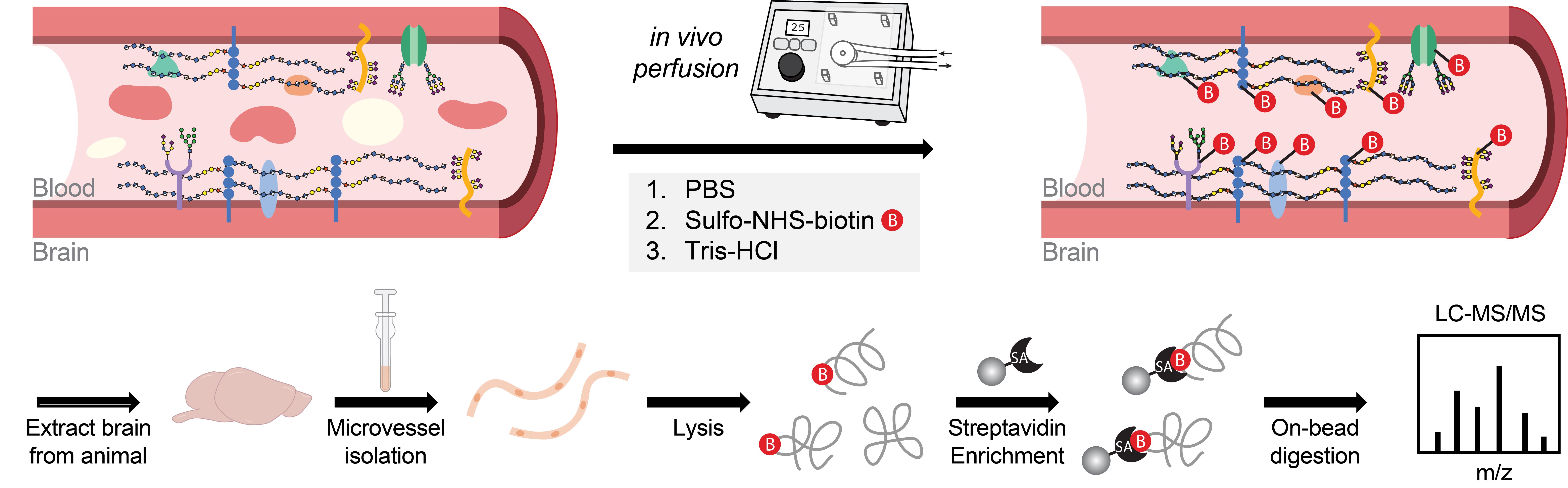
Luminal cerebrovascular proteomics workflow
Background
The cerebrovasculature plays a central role in maintaining brain homeostasis by tightly regulating the exchange of molecules between the blood and brain parenchyma. This function relies on the structural and functional polarization of brain endothelial cells, whose distinct luminal and abluminal surfaces mediate specialized interactions with the circulating milieu and neural microenvironment, respectively [1–3]. The luminal membrane serves as the primary interface with the bloodstream, harboring proteins that sense systemic signaling molecules, modulate vascular permeability, regulate immune surveillance, and support a distinct glycocalyx layer [3–5]. Despite its importance, previous cerebrovascular proteomic studies have often failed to capture the luminal proteome with high specificity, either identifying a limited number of luminal proteins or lacking the spatial resolution to distinguish between the luminal and abluminal compartments [6–8]. To address these limitations, we developed a perfusion-based protocol using a slow, peristaltic pump system to deliver a membrane-impermeable biotinylation reagent that selectively labels surface-exposed proteins on the luminal side of the cerebrovasculature. This is followed by vascular enrichment [9–11] and streptavidin-based protein capture for downstream protein identification via LC/MS–MS [12,13], enabling high-specificity isolation of luminal proteins with reduced background contamination and improved protein recovery. Using this approach, we identified over 1,000 luminal surface proteins from the brain vasculature in mice, the majority of which are annotated as cell surface proteins involved in key biologically relevant pathways such as transport, adhesion, communication, signaling, and extracellular matrix organization. This method offers a robust and scalable platform for in vivo mapping of the luminal cerebrovascular proteome and can be directly used for applications such as revealing biomarkers of vascular dysfunction and discovering new surface receptors for targeted CNS drug delivery. Furthermore, direct comparison of luminal-specific and whole-surface vascular proteomics provides a powerful approach to resolve the molecular distinctions between luminal and abluminal compartments and deepen our understanding of endothelial polarity in vivo.
Materials and reagents
Biological materials
1. C57BL/6J mice (Jackson Laboratory, catalog number: 000664)
Reagents
1. Phosphate-buffered saline (PBS) (Thermo Fisher Scientific, catalog number: 10010049)
2. HBSS, calcium, magnesium, no phenol red (Thermo Fisher Scientific, GibcoTM, catalog number: 14025092)
3. 2,2,2-tribromoethanol (Sigma-Aldrich, catalog number: T48402)
4. 2-methyl-2-butanol (Sigma-Aldrich, catalog number: 240486)
5. UltraPureTM 1 M Tris-HCl buffer, pH 7.5 (Thermo Fisher Scientific, catalog number: 15567027)
6. Bovine serum albumin (BSA) (Thermo Fisher Scientific, catalog number: 50-253-900)
7. Sulfo-NHS-Biotin (Thermo Fisher Scientific, catalog number: 21217)
8. cOmpleteTM, EDTA-free protease inhibitor (PI) cocktail (Thermo Fisher Scientific, catalog number: 11873580001)
9. Dextran from Leuconostoc mesenteroides (Millipore Sigma, catalog number: D8821)
10. RIPA lysis and extraction buffer (Thermo Fisher Scientific, catalog number: 89901)
11. PierceTM BCA Protein Assay kit (Thermo Fisher Scientific, catalog number: 23225)
12. PierceTM streptavidin magnetic beads (Thermo Fisher Scientific, catalog number: 88816)
13. Urea (Millipore Sigma, catalog number: 23225)
14. Iodoacetamide (IAA) (Millipore Sigma, catalog number: I1149)
15. Dithiothreitol (DTT) (Thermo Fisher Scientific, catalog number: R0861)
16. Trypsin/Lys-C Mix, mass spec grade (Promega, catalog number: V5073)
17. Water (LC–MS grade) (Thermo Fisher Scientific, catalog number: 51140)
18. Formic acid (LC–MS grade) (Thermo Fisher Scientific, catalog number: 28905)
19. Acetonitrile (LC–MS grade) (Thermo Fisher Scientific, catalog number: 51101)
20. Methanol (LC–MS grade) (Thermo Fisher Scientific, catalog number: A456)
Solutions
1. Avertin (see Recipes)
2. 1% BSA (see Recipes)
3. 1% BSA + 1× PI (see Recipes)
4. 32% Dextran (see Recipes)
5. 0.5 mg/mL Sulfo-NHS-biotin (see Recipes)
6. 50 mM Tris-PBS (see Recipes)
7. RIPA lysis buffer +1× PI (see Recipes)
8. 50mM Tris-HCl (pH 7.5) (see Recipes)
9. 2 M Urea in 50 mM Tris (see Recipes)
10. 2 M Urea in 50 mM Tris with 1 mM dithiothreitol (DTT) and 0.4 μg trypsin/LysC (see Recipes)
11. 0.1% Formic acid in LC–MS-grade water (solvent A) (see Recipes)
12. 0.1% Formic acid in 80% LC–MS-grade acetonitrile (solvent B) (see Recipes)
13. 0.1% Formic acid in LC–MS-grade acetonitrile (solvent Y) (see Recipes)
Recipes
1. Avertin (2.5% v/v)
Prepare in advance. 40× Avertin stocks are prepared prior by dissolving 10 g of 2,2,2-triboromoethanol in 10 mL of 2-methyl-2-butanol using gentle warming (~50 °C) and stirring in the dark. Store 1 mL aliquots of 40× Avertin at -80 °C for up to 12 months. Prepare 1× Avertin by mixing 1 mL of 40× Avertin with 39 mL of PBS. Store at 2–8 °C protected from light for up to 4 weeks.
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Avertin (40× stock) | 1× (2.5% v/v) | 1 mL |
| PBS | n/a | 39 mL |
| Total | n/a | 40 mL |
2. 1% BSA
Add 5 g of BSA to a sterile 500 mL bottle of 1× PBS and mix until dissolved. Store at 2–8 °C for up to 4 weeks.
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| BSA | 1% (w/v) | 5 g |
| PBS | n/a | 500 mL |
| Total | n/a | 500 mL |
3. 1% BSA + 1× PI
Prepare and aliquot 25× PI according to the manufacturer’s instructions. Thaw a vial of 25× PI and add 200 μL of 25× PI to 4,800 μL of 1% BSA in PBS. Prepare 5 mL per sample. Store on ice and make fresh.
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| 25× protease inhibitor | 1× | 200 μL |
| 1% BSA in PBS (Recipe 2) | n/a | 4,800 μL |
| Total | n/a | 5,000 μL |
4. 32% Dextran
Prepare in advance. Add HBSS to 100 g of Dextran from Leuconostoc mesenteroides for a final volume of 312.5 mL. Add a clean magnetic stirring rod and stir at room temperature until dissolved. Can be stored at 4 °C for up to 3 months.
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Dextran | 32% (w/v) | 100 g |
| HBSS | n/a | Fill to 312.5 mL |
| Total | n/a | 312.5 mL |
5. 0.5 mg/mL Sulfo-NHS-biotin
Add 10 mg of Sulfo-NHS-biotin to 20 mL of PBS. Vortex until dissolved. Prepare 20 mL of solution fresh per animal and use immediately.
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Sulfo-NHS-biotin | 0.5 mg/mL | 10 mg |
| PBS | n/a | 20 mL |
| Total | n/a | 20 mL |
6. 50 mM Tris-PBS
Add 0.5 mL of 1 M Tris-HCl buffer (pH 7.5) to 9.5 mL of PBS and vortex until dissolved. Prepare 10 mL of solution per animal. Store at room temperature for up to 1 week.
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| 1 M Tris-HCl buffer, pH 7.5 | 50 mM | 0.5 mL |
| PBS | n/a | 9.5 mL |
| Total | n/a | 10 mL |
7. RIPA lysis buffer + 1× PI
Prepare and aliquot 25× PI according to the manufacturer’s instructions. Thaw a vial of 25× PI and add 8 μL of 25× PI to 192 μL of RIPA lysis buffer. Prepare 200 μL per sample, plus additional volume for sample dilution as needed. Store on ice and make fresh.
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| 25× protease inhibitor | 1× | 8 μL |
| RIPA lysis buffer | n/a | 192 μL |
| Total | n/a | 200 μL |
8. 50 mM Tris-HCl (pH 7.5)
Add 35 μL of 1 M Tris-HCl buffer (pH 7.5) to 665 μL of LC–MS-grade water and vortex to mix. Prepare at least 700 μL per sample. Store at room temperature for up to 1 week.
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| 1 M Tris-HCl buffer, pH 7.5 | 50 mM | 35 μL |
| LC–MS-grade water | n/a | 665 μL |
| Total | n/a | 700 μL |
9. 2 M Urea in 50 mM Tris (pH 7.5)
Add 60.06 mg of urea to 500 μL of LC–MS-grade water and vortex to mix. Prepare fresh 500 μL of solution per sample.
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Urea | 2 M | 60.06 mg |
| 50 mM Tris-HCl | n/a | 500 μL |
| Total | n/a | 500 μL |
10. 2 M Urea in 50 mM Tris with 1 mM DTT and 5 μg/mL trypsin/Lys-C
Prepare 1 M stocks of DTT by dissolving 30.85 g of DTT in 20 mL of sterile water and store aliquoted stocks at -20 °C. Reconstitute trypsin/Lys-C according to manufacturer instructions at 0.2 μg/μL, and store aliquoted stocks at -80 °C. Add 0.08 μL of 1 M DTT and 2 μL of 0.2 μg/μL Trypsin/LysC to 78 μL of 2 M Urea in 50 mM Tris. Prepare 80 μL of solution fresh per sample.
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| 1 M DTT | 1 mM | 0.08 μL |
| 0.2 μg/μL Trypsin/LysC | 5 μg/mL | 2 μL |
| 2 M Urea in 50 mM Tris | n/a | 78 μL |
| Total | n/a | 80 μL |
11. 0.1% formic acid in LC–MS-grade water (solvent A)
Add 0.5 mL of formic acid to 499.5 mL of LC–MS-grade water and mix thoroughly. Store at room temperature for up to 6 months.
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| LC–MS-grade formic acid | 0.1% (v/v) | 0.5 mL |
| LC–MS-grade water | n/a | 499.5 mL |
| Total | n/a | 500 mL |
12. 0.1% formic acid in 80% LC–MS-grade acetonitrile (solvent B)
Mix 0.15 μL of formic acid, 29.85 μL of LC–MS-grade water, and 120 μL of LC–MS-grade acetonitrile. Prepare 150 μL per sample. Store at room temperature for up to 1 month.
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| LC–MS-grade formic acid | 0.1% (v/v) | 0.15 μL |
| LC–MS-grade water | n/a | 29.85 μL |
| LC–MS-grade acetonitrile | n/a | 120 μL |
| Total | n/a | 150 μL |
13. 0.1% formic acid in LC–MS-grade acetonitrile (solvent Y)
Add 0.5 mL of formic acid to 499.5 mL of LC–MS-grade acetonitrile and mix thoroughly. Store at room temperature for up to 6 months.
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| LC–MS-grade formic acid | 0.1% (v/v) | 0.5 mL |
| LC–MS-grade acetonitrile | n/a | 499.5 mL |
| Total | n/a | 500 mL |
Laboratory supplies
1. Corning® 50 mL centrifuge tubes (Millipore Sigma, catalog number: CLS4558-300EA)
2. Corning® 15 mL centrifuge tubes (Millipore Sigma, catalog number: CLS430790-500EA)
3. Terumo Surflo Winged Infusion Set 25G × 1/2” (Fisher Scientific, catalog number: 22-289911)
4. 30 mL syringe (Fisher Scientific, catalog number: BD302832)
5. Dissection tools
a. Iris scissors, 10 cm, SuperCut, straight, German (World Precision Instruments, catalog number: 14218-G)
b. Iris forceps, 10 cm, curved, serrated, German (World Precision Instruments, catalog number: 15915-G)
c. Student Halsted-mosquito hemostats (Fine Science Tools, catalog number: 91308-12)
6. Wheaton® 357424 glass 7 mL Tenbroeck Tissue Grinder Set, grinding chamber O.D. × L: 16 × 82 mm (Dounce homogenizer) (catalog number: 357424)
7. VWR® disposable Petri dishes, 60 × 15, mono Petri dishes (VWR International, catalog number: 25384-168)
8. VWR® razor blades (VWR International, catalog number: 55411-050)
9. Corning® cell strainer, pore size 40 μm (Corning, Millipore Sigma, catalog number: CLS431750-50EA)
10. Snap Cap low retention microcentrifuge tubes (Thermo Fisher Scientific, catalog number: 3448PK)
11. EppendorfTM Protein LoBindTM tubes (Thermo Fisher Scientific, catalog number: 13-698-794)
12. DynaMagTM-2 magnet (Thermo Fisher Scientific, catalog number: 12321D)
13. BioPureSPN mini C18 columns (The Nest Group, catalog number: HUM S18V)
Equipment
1. VWR® variable-speed peristaltic pump (VWR, catalog number: 70730-062)
2. Qsonica sonicator Q125 (Fisher Scientific, catalog number: 15-338-283)
3. Centrifuge 5810/5810 R (Eppendorf, catalog number/model: 022625004)
4. Centrifuge 5425 (Eppendorf, catalog number: 5405000042)
5. Pipettes
6. End-over-end tube rotator
7. LabconcoTM CentriVapTM benchtop concentrator (Fisher Scientific, catalog number: 16-108-334)
8. LC–MS/MS (Thermo Fisher Scientific, Q Exactive HF-X coupled with UltiMate 3000 RSLCnano system)
Software and datasets
1. MaxQuant (Max Planck Institute of Biochemistry, Version 1.6.10.43)
2. Perseus (Max Planck Institute of Biochemistry, Version 2.0.11.0)
Procedure
文章信息
稿件历史记录
提交日期: May 29, 2025
接收日期: Jul 10, 2025
在线发布日期: Jul 25, 2025
出版日期: Aug 5, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/).
如何引用
Shi, S. M., Bertozzi, C. R. and Wyss-Coray, T. (2025). Luminal Cerebrovascular Proteomics. Bio-protocol 15(15): e5411. DOI: 10.21769/BioProtoc.5411.
分类
神经科学 > 神经系统疾病 > 血脑屏障
系统生物学 > 蛋白质组学 > 空间蛋白组学
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link