- EN - English
- CN - 中文
Analyzing RNA Localization Using the RNA Proximity Labeling Method OINC-seq
基于RNA邻位标记的OINC-seq方法分析RNA定位
发布: 2025年08月05日第15卷第15期 DOI: 10.21769/BioProtoc.5403 浏览次数: 2374
评审: Alessandro DidonnaAnonymous reviewer(s)
Abstract
Thousands of RNAs are localized to specific subcellular locations, and these localization patterns are often required for optimal cell function. However, the sequences within RNAs that direct their transport are unknown for almost all localized transcripts. Similarly, the RNA content of most subcellular locations remains unknown. To facilitate the study of subcellular transcriptomes, we developed the RNA proximity labeling method OINC-seq. OINC-seq utilizes photoactivatable, spatially restricted RNA oxidation to specifically label RNA in proximity to a subcellularly localized bait protein. After labeling, these oxidative RNA marks are then read out via high-throughput sequencing due to their ability to induce predictable misincorporation events by reverse transcriptase. These induced mutations are then quantitatively assessed for each gene using our software package PIGPEN. The observed mutation rate for a given RNA species is therefore related to its proximity to the localized bait protein. This protocol describes procedures for assaying RNA localization via OINC-seq experiments as well as computational procedures for analyzing the resulting data using PIGPEN.
Key features
• OINC-seq assays the RNA content of a variety of subcellular locations.
• OINC-seq utilizes a photoactivatable, proximity-dependent RNA oxidation reaction to label RNAs.
• Oxidative RNA marks are read using high-throughput sequencing without the need for enrichment.
• Oxidative RNA marks are identified and quantified using the associated PIGPEN software.
Keywords: Proximity labeling (邻位标记)Graphical overview
Background
In a variety of organismal and developmental contexts, the localization of specific RNAs to specific subcellular locations promotes cellular and organismal function. For example, mate type switching in yeast [1] and developmental patterning in Drosophila [2,3] are controlled in part through the trafficking of specific mRNAs to precise subcellular locations. Mutations in RNA-binding proteins involved in RNA transport are associated with a variety of human neurological diseases [4,5]. However, for most localized RNAs, the mechanisms that govern their transport remain unknown. This includes the cis-elements within transcripts that mark them as RNAs to be localized, as well as the RNA-binding proteins (RBPs) that mediate the process.
A common experimental technique in the study of RNA localization is the characterization of RNA populations at subcellular locations. Historically, this has often been achieved through cellular fractionation, either by biochemical or mechanical means, followed by RNA isolation and characterization from the resulting fractions [6–12]. Although these techniques have led to many insights, they also have disadvantages. Mechanical cellular fractionations often require that the interrogated cell type have specific morphologies. For example, neurons are commonly separated into soma and neurite fractions, but the technique for doing so relies on cells having long, thin projections [7]. Biochemical fractionations, although more flexible regarding cell shape and morphology, have their own challenges. The association between an RNA and a subcellular location or organelle may be weak or transient and therefore lost during purification, leading to false negatives. Similarly, RNA molecules in subcellular environments that are spatially distinct within intact cells may nevertheless have similar biochemical properties and therefore copurify, leading to false positives.
Newer techniques that rely on proximity labeling remove many of these concerns. In these approaches, bait proteins are first localized to the subcellular location of interest [13–16]. Upon a chemical or light-based stimulus, RNAs in close proximity to the bait protein (usually 20–100 nm) are specifically labeled. In the majority of the techniques reported to date, these labels result in the biotinylation of bait-proximal RNAs, facilitating their downstream isolation with streptavidin and characterization.
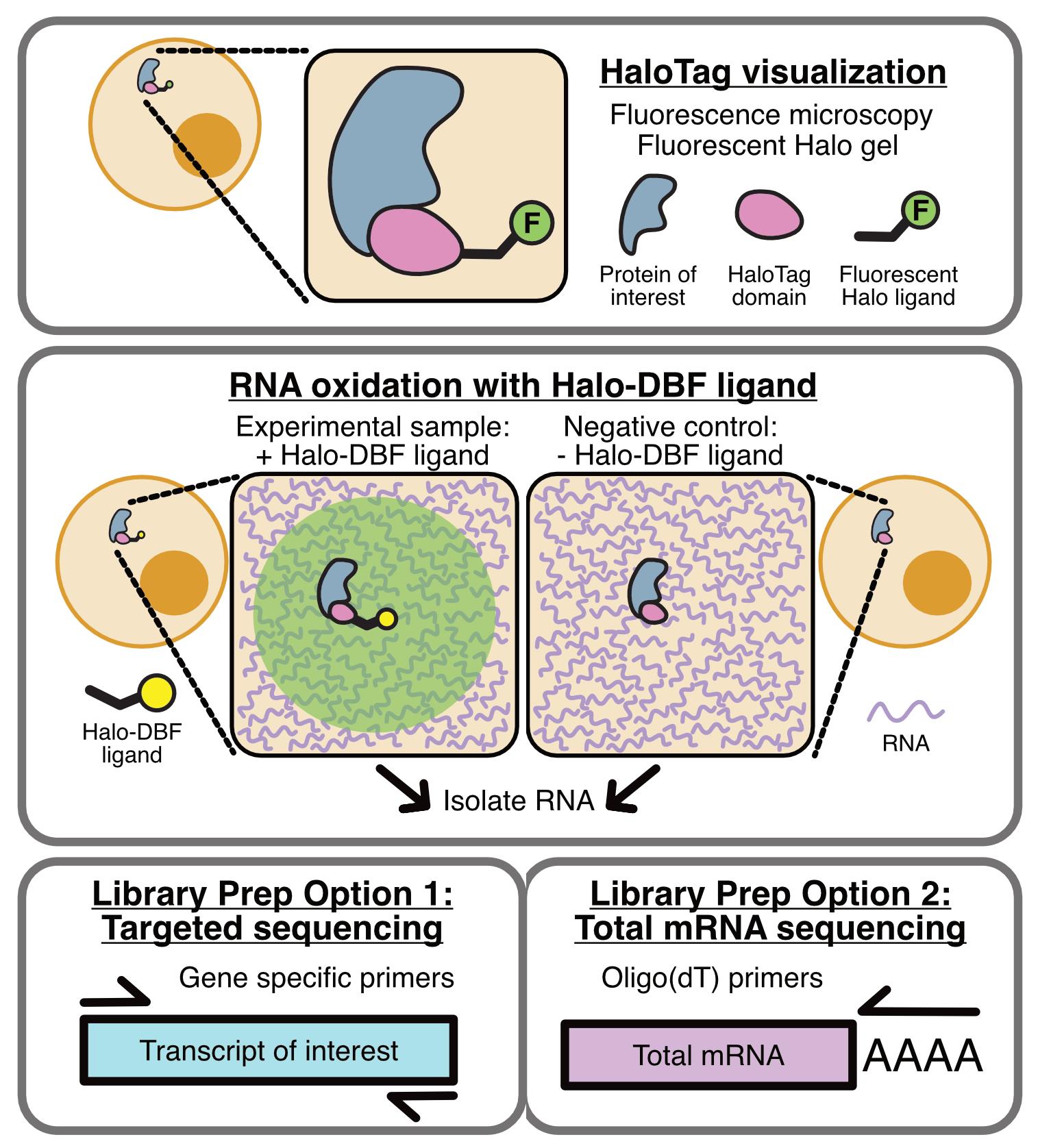
OINC-seq builds on one of these RNA proximity labeling methods, Halo-seq [13,17]. In Halo-seq, a HaloTag-fused protein is specifically localized to the subcellular location of interest [18]. HaloTag domains covalently bind to Halo ligands. Upon addition of Halo-DBF ligand, a dibromofluorescein (DBF) molecule therefore assumes the same subcellular distribution as the Halo-tagged bait protein. When irradiated with green light, DBF emits singlet oxygen radicals that oxidize nearby RNAs. Because guanosine residues have the lowest redox potential of any of the four bases, they are preferentially oxidized to form 8-oxoguanosine as well as further oxidized hydantoins.
In Halo-seq, these oxidized guanosine residues are then alkynylated, which makes them substrates for biotinylation through Click chemistry. With OINC-seq, however, we sought to streamline the process to detect oxidized guanosines directly without the need for alkynylation, biotinylation, and purification. Instead, following the light-induced oxidation of bait-proximal RNA, total RNA is isolated and reverse transcribed. Reverse transcriptase predictably misinterprets oxidized guanosine [19], leading to G to T, G to C, and G deletion mutations in the resulting cDNA. Quantification of these mutations via high-throughput sequencing therefore provides a readout of the relative proximity of each RNA species to the Halo-tagged bait protein. In order to identify and quantify these mutations in RNAseq data, we developed the PIGPEN software. PIGPEN takes in RNAseq reads from an OINC-seq experiment and returns tables of mutation rates for each gene detected in the sample.
In general, OINC-seq experiments can take one of two forms. They can either be designed to assay the entire transcriptome, or they can be focused on one RNA through the interrogation of an amplicon. Although limited in focus to one RNA species, amplicon-based experiments typically have extensive read depth for the RNA of interest, leading to more accurate results. In contrast, although transcriptome-wide experiments interrogate thousands of RNA species at once, the reduced read depth assigned to any one RNA species can lead to noisier results.
Oxidation-induced mutations are relatively rare. This rarity requires that other, non-oxidation-derived sources of mutations be suppressed as much as possible. For example, Illumina sequencers miscall approximately 1 out of 1,000 bases in a typical sequencing reaction. In principle, it is impossible to distinguish between such a miscall and an oxidation-induced mutation. The usage of paired-end reads can mitigate this by requiring that any given mutation be seen in both reads of a mate pair in order to be classified as a true mutation and not a product of sequencer error. Specific options in PIGPEN implement this functionality.
RNA in close proximity to the bait protein may be expected to have multiple oxidation events and, therefore, multiple mutations along the same molecule. Random mutations, on the other hand, may be expected to be more evenly distributed across the entire RNA population. The appearance of multiple mutations within the same read might then give additional confidence in the localization of an RNA. Options in PIGPEN also support this functionality.
Finally, because of the rarity of oxidation-induced mutations, the fidelity of the polymerases used in the making of RNAseq libraries also matters. During the development of OINC-seq, we tested multiple library preparation kits and found that Quantseq kits from Lexogen consistently had the lowest background mutation rate in untreated samples, making them ideal for use with OINC-seq. However, these kits only interrogate the 3’ ends of molecules with poly-A tails. RNAs without these tails are therefore not assayable using this kit. It is possible that other library preparation methods would have suitable rates of background mutations, but they have not yet been tested. Amplicon-based experiments afford more flexibility in polymerase choice. We have found that the use of SuperScript IV for reverse transcription and NEB Q5 polymerase for PCR consistently leads to acceptably low levels of background mutations.
Materials and reagents
Biological materials
1. Cell line expressing HaloTag fusion protein
Note: In this protocol, authors use HeLa cells (ATCC, catalog number: CCL-2).
Reagents
1. Cell culture medium specific to the cell line used. In this protocol, the authors used the following formulation for HeLa cell culture: DMEM + D-Glucose + L-Glutamine (Gibco, catalog number: 11965-092), 10% EqualFETAL serum (Atlas Biologicals, catalog number: EF-0500-A), and 1% penicillin-streptomycin (Gibco, catalog number: 15140-122)
2. Doxycycline hydrochloride (Fisher Scientific, catalog number: AAJ6042203v)
3. Phosphate-buffered saline (PBS) (Invitrogen, catalog number: AM9625)
4. Hank’s balanced salt solution (HBSS) without calcium or phenol red (VWR, catalog number: VWRL0121-0500)
5. Janelia Fluor 549 HaloTag Ligand (Promega, catalog number: HT1020; product format: 100 μM in nuclease-free water)
6. Formaldehyde, 37% by weight (Fisher Scientific, catalog number: BP531-500)
7. DAPI (Sigma-Aldrich, catalog number: D9542-1MG; product format: 25 μM in nuclease-free water)
8. Fluoromount-G (Southern Biotech, catalog number: 0100-01)
9. Nail polish
10. 5 M NaCl (Quality Biological, catalog number: 351-036-1)
11. 0.5 M EDTA (Invitrogen, catalog number: AM9261)
12. 1 M Tris (Affymetrix, catalog number: 22638)
13. Igepal CA-630 (MP Biomedicals, catalog number: MFCD00132506)
14. Sodium deoxycholate (VWR, catalog number: 0613-50G)
15. SDS (VWR, catalog number: 0227-100G)
16. NuPAGE LDS sample buffer 4× (Invitrogen, catalog number: NP0008)
17. Dithiothreitol (DTT) (Gold Bio, catalog number: DTT10)
18. Spectra multicolor broad range protein ladder (Invitrogen, catalog number: 26634)
19. NuPAGE Bis-Tris Mini protein gels, 4%–12%, 1.0 mm (Invitrogen, catalog number: NP0321BOX)
20. NuPAGE MOPS SDS running buffer 20× (Invitrogen, catalog number: NP0001)
21. Coomassie Brilliant Blue R-250 dye (G-Biosciences, catalog number: 786-495)
22. Methanol (VWR, catalog number: BDH1135-4LP)
23. Acetic acid (VWR, catalog number: 20104.312)
24. 5 mM Halo-DBF in DMSO; store at -20 °C protected from light and water [20]
Note: This is not available commercially, but synthesis instructions are provided in the above reference.
25. Trizol (Invitrogen, catalog number: 15596018)
26. Nuclease-free water (Invitrogen, catalog number: AM9937)
27. DNase I (New England Biolabs, catalog number: M0303S)
28. SuperScript IV reverse transcriptase (Invitrogen, catalog number: 18090010)
29. RNase H (New England Biolabs, catalog number: M0297S)
30. RNase A/T1 (Thermo Scientific, catalog number: EN0551)
31. Q5 High-Fidelity 2× master mix (New England Biolabs, catalog number: M0492L)
32. DNA Clean and Concentrator-5 (Zymo Research, catalog number: D4014)
33. KAPA Pure Beads (Roche, catalog number: 07983271001)
34. QuantSeq 3’ mRNA-Seq Library Prep Kit FWD (Lexogen, catalog number: O15)
35. 1 M Tris pH 8.0 (Thermo Fisher Scientific, catalog number: AAJ22638K2)
36. Qubit RNA HS Assay kit (Thermo Fisher Scientific, catalog number: Q32855)
Solutions
1. Halo-JF buffer (see Recipes)
2. Formaldehyde fixation buffer (see Recipes)
3. RIPA (see Recipes)
4. Coomassie Brilliant Blue stain (see Recipes)
5. Coomassie destain (see Recipes)
6. Halo-DBF buffer (see Recipes)
7. DAPI buffer (see Recipes)
8. RNase buffer (see Recipes)
Recipes
1. Halo-JF buffer
Halo-JF buffer should be prepared fresh for each experiment.
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Janelia Fluor Halo ligand | 50 nM | 0.5 μL |
| HBSS | 100% | 1 mL |
2. Formaldehyde fixation buffer
Fixation buffer should be prepared fresh for each experiment.
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| 37% Formaldehyde | 3.7% | 100 μL |
| PBS | 90% | 900 μL |
3. RIPA
RIPA can be stored at 4 °C for 6 months.
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| 5 M NaCl | 150 mM | 3 mL |
| 0.5 M EDTA, pH 8.0 | 5 mM | 1 mL |
| 1 M Tris, pH 8.0 | 50 mM | 5 mL |
| NP-40 (IGEPAL CA-630) | 1% | 1 mL |
| 10% sodium deoxycholate | 0.5% | 5 mL |
| 10% SDS | 0.1% | 1 mL |
| dH2O | 84 mL |
4. Coomassie Brilliant Blue stain
Coomassie Brilliant Blue stain can be stored at room temperature for 6 months.
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Coomassie R250 dye | 0.1% (w/w) | 1 g |
| Methanol | 30% | 300 mL |
| Acetic acid | 5% | 50 mL |
| MilliQ H2O | 650 mL |
5. Coomassie destain
Coomassie destain can be stored at room temperature for 6 months.
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Methanol | 30% | 300 mL |
| Acetic acid | 5% | 50 mL |
| MilliQ H2O | 650 mL |
6. Halo-DBF buffer
Halo-DBF buffer should be prepared fresh for each experiment.
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Halo-DBF ligand | 5 nM | 1 μL |
| HBSS | 100% | 1 mL |
7. DAPI Buffer
DAPI buffer should be prepared fresh for each experiment.
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| DAPI | 100 ng/mL | 4 μL |
| PBS | 100% | 1 mL |
8. RNase buffer
RNase buffer should be prepared fresh for each experiment.
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| RNase H | 50% | 1 μL |
| RNase A/T1 | 50% | 1 μL |
Laboratory supplies
1. 12-well tissue culture plate (Cell Treat, catalog number: 229111)
2. 10 cm tissue culture dish (USA Scientific, catalog number: CC7682-3394)
3. Poly-d-Lysine (PDL)-coated coverslips (NeuVitro, catalog number: GG-12-15-PDL)
4. Microscope slides (Globe Scientific, catalog number: 1380-20)
5. Quick-RNA Miniprep kit (Zymogen Research, catalog number: R1055)
6. Cell scraper/lifter (Fisher Scientific, catalog number: 07-200-364)
7. 1.5 mL microcentrifuge tubes (Eppendorf, catalog number: 07-200-364)
8. 1 mL Luer-lock syringes (Air-Tite, catalog number: ML-1)
9. 20 G 1.5 in. syringes (BD, catalog number: 305176)
10. 0.2 mL PCR strip tubes (Light Labs, catalog number: A-40030A)
11. Polarized laboratory glasses (Bolle, catalog number: BOLBAXPOLWFS)
Equipment
1. Biosafety cabinet (Thermo Fisher Scientific, catalog number: 1335)
2. Cell culture incubator (Thermo Fisher Scientific, catalog number: 370)
3. Bead bath (Lab Armor, catalog number: 74300-720)
4. Tweezers (Electron Microscopy Sciences, catalog number: 78326-51)
5. Fluorescence microscope (3i, model: Marianas)
6. Tube racks
7. Microcentrifuge (Eppendorf, catalog number: 5424)
8. Refrigerator (4 °C)
9. Freezer (-20 °C)
10. Ultra-low freezer (-80 °C)
11. Nanodrop spectrophotometer (Thermo Fisher Scientific, catalog number: ND-ONE-W)
12. Thermocycler (Bio-Rad, model: C1000 Touch)
13. Biomolecular Imager (Azure Biosystems, model: Sapphire)
14. Green LED flood lights (T-SUN, purchased from Amazon.com, catalog number: TS-F4250-RGBCW-US-2Pack)
15. DynaMag-2 magnet (Invitrogen, catalog number: 12321D)
16. Qubit 3 fluorometer (Thermo Fisher Scientific, catalog number: Q33216)
17. 4150 TapeStation (Agilent, catalog number: G2992AA)
Software and datasets
1. PIGPEN software, for analysis of OINC-seq experiments, is freely available for download here: https://github.com/TaliaferroLab/OINC-seq.
Procedure
文章信息
稿件历史记录
提交日期: May 9, 2025
接收日期: Jun 24, 2025
在线发布日期: Jul 22, 2025
出版日期: Aug 5, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Pockalny, M. C., Lo, H.-Y. G., Goering, R. and Taliaferro, J. M. (2025). Analyzing RNA Localization Using the RNA Proximity Labeling Method OINC-seq. Bio-protocol 15(15): e5403. DOI: 10.21769/BioProtoc.5403.
分类
生物信息学与计算生物学
分子生物学 > RNA > RNA定位
分子生物学 > RNA > RNA 测序
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link












