- EN - English
- CN - 中文
A Step-By-Step Protocol for Correlative Light and Electron Microscopy Imaging of Proteinaceous Deposits in Cultured Cells and Human Brain Tissues
细胞培养与人脑组织中蛋白质沉积物的光电相关显微成像操作步骤详解
发布: 2025年08月05日第15卷第15期 DOI: 10.21769/BioProtoc.5402 浏览次数: 2335
评审: David PaulAnonymous reviewer(s)
Abstract
An improved correlative light and electron microscopy (CLEM) method has recently been introduced and successfully employed to identify and analyze protein inclusions in cultured cells as well as pathological proteinaceous deposits in postmortem human brain tissues from individuals with diverse neurodegenerative diseases. This method significantly enhances antigen preservation and target registration by replacing conventional dehydration and embedding reagents. It achieves an optimal balance of sensitivity, accuracy, efficiency, and cost-effectiveness compared to other current CLEM approaches. However, due to space constraints, only a brief overview of this method was provided in the initial publication. To ensure reproducibility and facilitate widespread adoption, the author now presents a detailed, step-by-step protocol of this optimized CLEM technique. By enhancing usability and accessibility, this protocol aims to promote broader application of CLEM in neurodegenerative disease research.
Key features
• This protocol incorporates optimized sample processing and innovative fiducial marking techniques that enhance antigen preservation and improve target registration, respectively.
• By utilizing serial ultrathin sections for CLEM, this protocol significantly increases correlation accuracy.
• A novel “sandwich method” is introduced, which enables simultaneous detection of multiple proteinopathies through immunofluorescence staining or precise localization of pathological targets using immunogold labeling.
• Overall, this protocol offers an effective balance of sensitivity, accuracy, efficiency, and cost-effectiveness compared to existing CLEM methodologies.
Keywords: Correlative light and electron microscopy (光电相关显微镜)Graphical overview
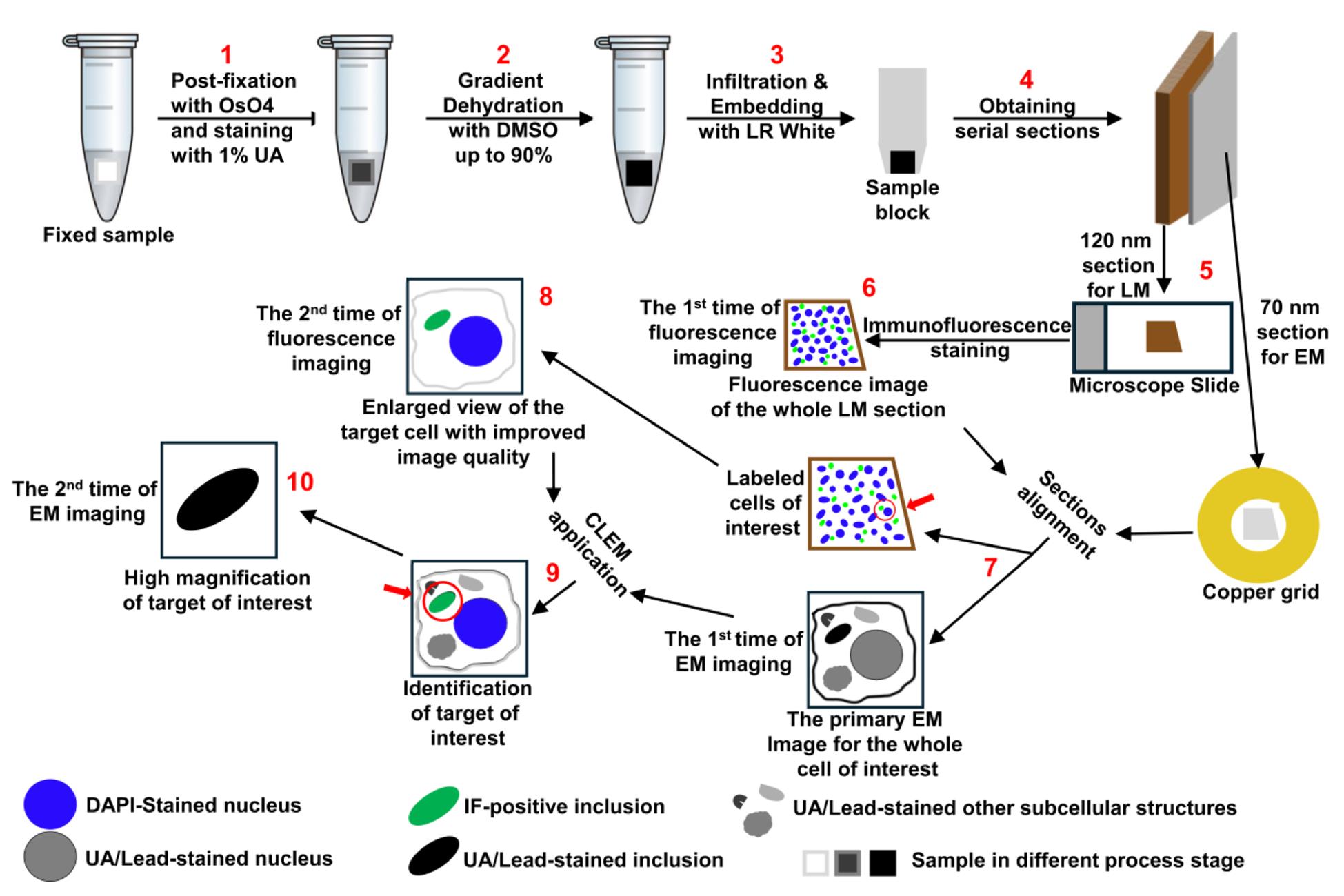
Background
Although protein deposits are a common feature of many neurodegenerative diseases, their distribution and primary components can vary significantly between different disease types [1,2]. Moreover, the size, quantity, and spreading patterns of these deposits in the human brain are often closely associated with disease severity [3–5]. Despite this, the underlying causes of these diseases remain largely unclear. For accurate diagnosis, prevention, and therapeutic development, it is crucial to understand the mechanisms driving the formation and progression of protein deposits in the human brain [6–8]. Achieving this depends heavily on our ability to identify neuropathological protein deposits, characterize their ultrastructural features, and determine their associated components [2,9–12]. In this context, correlative light and electron microscopy (CLEM), a technique that combines the strengths of light microscopy and electron microscopy to provide complementary information across a broad resolution range (~250 to ~0.1 nm) [13–15], has proven to be a powerful tool. By enabling the examination of the same target at both microstructural and ultrastructural levels, CLEM greatly enhances the accuracy of interpreting structural and molecular features [11,12,16–19]. However, current CLEM methods are often technically demanding and logistically complex, limiting their routine use in standard research laboratories [20]. To overcome these challenges, the author has developed a simplified, time-efficient CLEM protocol, which has been successfully applied to the identification and analysis of proteinaceous inclusions in both cell model and postmortem human brain tissue [20]. The step-by-step protocol for this optimized CLEM technique is presented below. With its superior antigen preservation and target registration compared to existing methods, this protocol is expected to broaden access to CLEM and facilitate advancements in research on neurodegenerative diseases and related fields.
Materials and reagents
Biological materials
1. Cultured cells: A cell model derived from the H4 neuroglioma line (H4/αS-HoTag3) was used. This model generates α-synuclein (αS) inclusions through stable expression of recombinant αS fused to HoTag3 at the C-terminus, making it ideal for demonstration of CLEM application in cell samples to reveal ultrastructural details of protein aggregates [20]. This cell model is available from the author’s laboratory upon request.
2. Human brain tissues: Post-mortem brain tissues from patients with confirmed neurodegenerative diseases were obtained from the Brain Bank for Neurodegenerative Disorders at Mayo Clinic Florida, with consent and institutional approval.
Reagents
1. Dimethyl sulfoxide (DMSO) (Fisher BioReagents, catalog number: BP231-1)
2. LR white (medium grade) (Electron Microscopy Sciences, catalog number: 14381)
3. Sodium meta-periodate (Thermo Fisher, catalog number: 20504)
4. VECTASHIELD® antifade mounting medium with DAPI (Vector Laboratories, catalog number: H-1200-10)
5. Toluidine blue O (VWR, catalog number: IC15264925)
6. Sodium borate 10-hydrate (Na2B4O7·10H2O) (The Science Company, catalog number: NC-1686)
7. Normal goat serum (Thermo Fisher, catalog number: 10000C)
8. Rabbit polyclonal antibody against α-synuclein (Mayo Clinic, catalog number: NACP98)
9. Goat anti-rabbit IgG (H+L) secondary antibody, Alexa FluorTM 488 (Thermo Fisher, catalog number: A-11034)
10. Gold-conjugated goat anti-rabbit IgG (H+L) secondary antibodies (12 nm) (Jackson Immuno Research, catalog number: 111-205-144)
11. Sodium cacodylate buffer, 0.4 M, pH 7.2 (Electron Microscopy Sciences, catalog number: 11655)
12. Glutaraldehyde, 10% in H2O (Electron Microscopy Sciences, catalog number: 16120)
13. Paraformaldehyde 16% aqueous solution, EM grade (Electron Microscopy Sciences, catalog number: 15710)
14. 2% osmium tetroxide aqueous solution (OsO4) (Electron Microscopy Sciences, catalog number: 19192)
15. TBS with TweenTM (TBST) (Thermo Scientific Chemicals, catalog number: J77500.K2)
16. 4% uranyl acetate solution (Electron Microscopy Sciences, catalog number: 22400-4)
17. Lead citrate, 3% solution (Electron Microscopy Sciences, catalog number: 22410-01)
18. Gelatin solution 0.1% (Lifeline Cell Technology, catalog number: 102970-804)
19. Sudan Black B Stain kit (ENG Scientific, catalog number: 4460)
Caution: Sodium cacodylate, glutaraldehyde, osmium tetroxide, uranyl acetate, lead citrate, and LR white are toxic chemicals. Always follow standard safety practices to handle and discard these substances.
Solutions
1. 1% toluidine blue in 1% borate (see Recipes)
2. Fixation solution (see Recipes)
3. 1% OsO4 (see Recipes)
4. 2% uranyl acetate solution (see Recipes)
Recipes
1. 1% toluidine blue in 1% borate
Dissolve 1 g of Na2B4O7·10H2O in approximately 80 mL of distilled water in a beaker. Add 1 g of toluidine blue O into the borate solution while stirring. Stir the solution until completely dissolved. Filter the solution through a paper filter (Whatman, 1001-918) if particulates remain. Adjust the pH to make sure it is around 9–10, which is suitable for staining. Add distilled water to bring the final volume to 100 mL. Store at room temperature. Label with date and concentration.
2. Fixation solution
Add 0.25 mL of 10% glutaraldehyde, 12.5 mL of 16% paraformaldehyde, and 12.5 mL of 0.4 M sodium cacodylate buffer into 24.5 mL of ultrapure water in a 50 mL high-clarity conical centrifuge tube. Adjust the final volume to 50 mL and mix thoroughly. Store at 4 °C. Avoid light or cover with aluminum foil.
| Reagent | Final concentration | Quantity (for 50 mL) |
|---|---|---|
| 10% glutaraldehyde | 0.05% | 0.25 mL |
| 16% paraformaldehyde | 4% | 12.5 mL |
| 0.4 M sodium cacodylate buffer | 0.1 M | 12.5 mL |
3. 1% OsO4
Add 5 mL of 2% OsO4 into 5 mL of ultrapure water and mix completely. Always prepare freshly.
4. 2% uranyl acetate solution
Add 5 mL of 4% uranyl acetate solution into 5 mL of ultrapure water and mix completely. Store at room temperature. Avoid light or cover with aluminum foil.
Laboratory supplies
1. General lab consumables: Gloves, pipettes, mask, paper towel, microcentrifuge tubes, plastic beakers, parafilm
2. Filter paper (Whatman, catalog numbers: 1002-090 and 1001-918)
3. Plastic Petri dish, 100 mm × 25 mm (Sigma, catalog number: Z358762)
4. BEEM® capsules (Polysciences, catalog number: 00224-500, size 00)
5. Lab microscope slides (Fisher Scientific, catalog number: 12-550-107)
6. 50 mL high-clarity conical centrifuge tubes (Corning, catalog number: 352070)
7. Dry ice
8. Formvar support film single hole 1,500 μm Cu grids (Electron Microscopy Sciences, catalog number: FFGA1500-Cu-50)
9. Formvar support film single hole 1,500 μm nickel grids (Electron Microscopy Sciences, catalog number: FFGA1500-Ni-50)
10. Coverslip forceps (Dumont, catalog number: 11251-33, #5/45)
11. Single edge blade (Uline, catalog number: H-595B)
12. Perfect handle & loop set (Electron Microscopy Sciences, catalog number: 70944)
13. Diamond knife ultra 45 °C, 2 mm (DIATOME, catalog number: DU4520)
14. 100-capacity grid storage box (Electron Microscopy Sciences)
Software and datasets
1. PhotoPad Image Editor (NCH Software)
2. Adobe Photoshop (Adobe)
Equipment
1. Benchtop centrifuge (Fisher Scientific, catalog number: 13-100-678, model: accuSpin 21R)
2. Vacuum oven (Fisher Scientific, catalog number: 13-262-50, Isotemp Model 281A Vacuum Oven)
3. Ultramicrotome (Leica Microsystems, model: Leica UC7)
4. Light microscope (Olympus, model: BX50)
5. Confocal laser-scanning microscope (ZEISS Microscopy, model: LSM 880)
6. Electron microscope (JEOL, model: JEM-1400 FLASH)
7. PELCO® UVC2 Cryo Chamber (Ted Pella, catalog number: 6202)
8. Rotator (Benchmark Roto-Mini Rotator, model: R2020)
Procedure
文章信息
稿件历史记录
提交日期: May 8, 2025
接收日期: Jun 24, 2025
在线发布日期: Jul 15, 2025
出版日期: Aug 5, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Jiang, P. and Dickson, D. W. (2025). A Step-By-Step Protocol for Correlative Light and Electron Microscopy Imaging of Proteinaceous Deposits in Cultured Cells and Human Brain Tissues. Bio-protocol 15(15): e5402. DOI: 10.21769/BioProtoc.5402.
分类
神经科学 > 神经系统疾病 > 神经退行性病变
神经科学 > 神经解剖学和神经环路 > 脑神经
细胞生物学 > 细胞成像 > 电子显微镜
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link