- EN - English
- CN - 中文
Reliable and Sensitive Detection of Carbonylated Proteins by Oxime Blot
氧肟印迹法实现羰基化蛋白的可靠灵敏检测
发布: 2025年08月05日第15卷第15期 DOI: 10.21769/BioProtoc.5401 浏览次数: 1235
评审: Alessandro DidonnaAnonymous reviewer(s)

相关实验方案
在平板检测仪中使用高特异性荧光探针定量巨噬细胞细胞二价铁 (Fe2+) 含量
Philipp Grubwieser [...] Christa Pfeifhofer-Obermair
2024年02月05日 2212 阅读
哺乳动物线粒体和胞质顺乌头酸酶的凝胶内活性测定——分区特异性氧化应激与铁状态的替代标志物
Wing-Hang Tong and Tracey A. Rouault
2024年12月05日 2199 阅读
Abstract
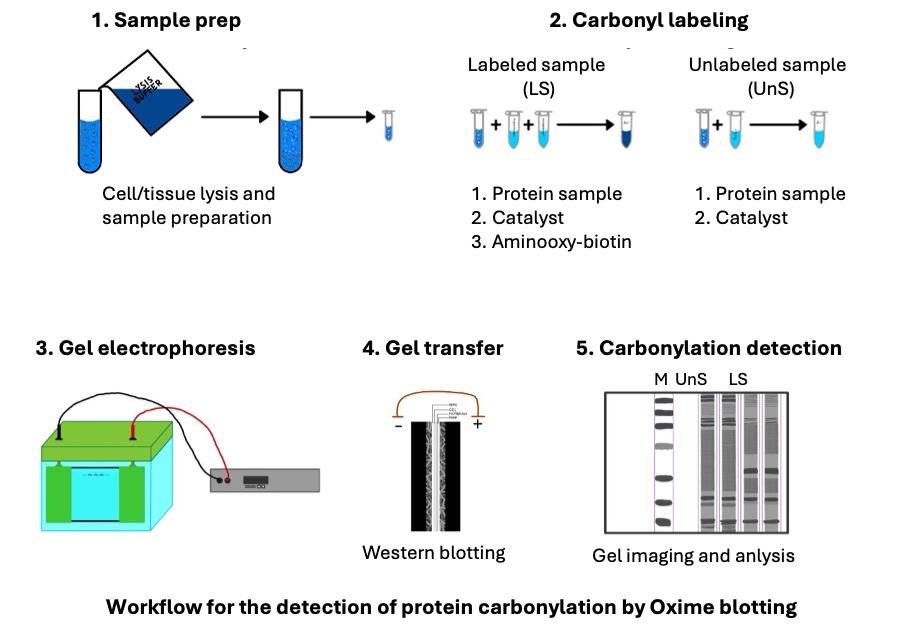
Oxidative protein damage is important in various biological processes and age-related diseases. Protein carbonylation is the predominant and most frequently studied form of protein oxidation. It is most frequently detected following its derivatization with 2,4-dinitrophenylhydrazine (DNPH) hapten, followed by its detection with an anti-DNP antibody. However, when used to detect protein carbonylation by western blotting, this method suffers from diminished sensitivity, distortion of protein migration patterns, and unsatisfactory representation of low-abundance proteins. This is due to the poor solubility of DNPH in typical buffer solutions, the acidic protein precipitation due to the use of strong acid for its dissolution, the instability in solution, and the distorted protein migration patterns introduced by an additional salt content generated by the required pH adjustment prior to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). To address the DNPH method limitations, a new Oxime blot technique was developed. This method is based on forming the stable oxime bonds between the protein carbonyl groups and biotin-aminooxy probe in the presence of a p-phenylenediamine (pPDA) catalyst at neutral pH conditions. The derivatization reaction reaches a plateau within 3 h. It ensures efficient and complete derivatization of carbonylated proteins, which are separated by SDS-PAGE without additional manipulation and detected with avidin-HRP and enhanced chemiluminescence (ECL) in western blotting. The Oxime blot protocol allows researchers to reliably and sensitively detect carbonylated proteins and provides a valuable tool for studying oxidative stress in diverse biological settings.
Key features
• This method enables the sensitive and reliable detection of protein carbonylation in various biological samples.
• The chemically stable oxime bond forms quickly and efficiently, reaching its plateau level after 3 h, enabling relative carbonylation quantification.
• Carbonylation derivatization at low salt content and neutral pH ensures good SDS-PAGE protein migration without any protein loss.
• This method integrates well with detecting specific protein carbonylation following its immunoprecipitation.
Keywords: Oxidative stress (氧化应激)Graphical overview
Background
Reversible protein oxidation is essential in the redox signaling pathways that regulate various physiological processes [1,2]. However, irreversible protein oxidation is much more prevalent. It could impair protein functions and contribute to cellular damage, aging, and age-related diseases such as cardiovascular, neurodegenerative, autoimmune diseases, cancer, cataracts, and type 2 diabetes. It occurs when antioxidant defense systems fail to neutralize the reactive oxygen species (ROS) generated during normal oxidative metabolism or under oxidative stress conditions. This stress can arise from endogenous factors (e.g., inflammation, ischemia-reperfusion injury) or exogenous factors (e.g., radiation, smoking, pollution, psychological stress). There are several forms of oxidative protein damage. The most prominent and widely studied form is protein carbonylation. This irreversible oxidative modification affects amino acid side chains or protein backbones [3–6].
Accurately detecting and measuring protein carbonylation is essential to understanding the role of protein carbonylation in cell biology and age-related diseases [7,8]. The most widely used method relies on carbonyl derivatization with 2,4-dinitrophenylhydrazine (DNPH). The resulting bioconjugate is typically detected with an anti-DNP antibody in enzyme-linked immunosorbent assay (ELISA) or a blot analysis [9,10]. However, DNPH methods have several challenges, such as poor reagent solubility, instability in solution, limited derivatization of carbonyl groups, inadequate representation of the low-abundance proteins, acidic precipitation-induced protein loss, and the need to adjust the pH prior to SDS-PAGE analysis, which could distort protein migration patterns due to the increased salt content. These issues can lead to questionable reliability and reproducibility [10–12].
We developed an alternative method to address these limitations using an aminooxy probe that forms stable oxime bonds with carbonyl groups. This reaction is accelerated by the catalyst p-phenylenediamine (pPDA) and is conducted at neutral pH [13–15]. The carbonyl derivatization with aminooxy reaches the reaction plateau within 3 h, enabling sensitive and reliable carbonylated protein detection. We named this detection method the Oxime blot method [16]. This protocol describes a step-by-step approach to detecting the carbonylation of purified protein and proteins from complex samples such as cell lysates. This protocol has the potential to facilitate more reliable and frequent investigations of protein carbonylation in various cell biology processes and the molecular mechanisms of diseases.
Materials and reagents
Biological materials
1. A549 cell line (ATCC, catalog number: CRL-1555)
Reagents
1. Dulbecco’s modified Eagle’s medium (DMEM), high glucose (Sigma-Aldrich, catalog number: D6429)
2. L-glutamine (200 mM) (Pan Biotech, catalog number: P04-80100)
3. Penicillin-streptomycin (5,000 U/mL) (Pan Biotech, catalog number: P06-07100)
4. Fetal bovine serum (FBS) (Pan Biotech, catalog number: P30-3306)
5. Trypsin EDTA solution (Pan Biotech, catalog number: P10-020100)
6. Dulbecco’s phosphate-buffered saline (DPBS) (Pan Biotech, catalog number: P04-35500)
7. Tris base (Sigma-Aldrich, catalog number: 252859)
8. cOmpleteTM Mini, EDTA-free protease inhibitor cocktail tablets (Sigma-Aldrich, catalog number: 11836170001)
9. Glycine (Sigma-Aldrich, catalog number: 33226)
10. Sodium dodecyl sulfate (SDS) (Carl Roth, catalog number: 0183.2)
11. Tween®-20 (Sigma-Aldrich, catalog number: P-1379)
12. NP-40 as TERGITOLTM solution (Sigma-Aldrich, catalog number: NP40S)
13. Sodium deoxycholate (Roth, catalog number: 3484.2)
14. Aprotinin (Sigma-Aldrich, catalog number: A3428)
15. Leupeptin (Sigma-Aldrich, catalog number: L0649)
16. Sodium fluoride (NaF) (Sigma-Aldrich, catalog number: S1504)
17. Sodium vanadate (Na3VO4) (Sigma-Aldrich, catalog number: S6508)
18. Phenylmethylsulfonyl fluoride (PMSF) (Sigma-Aldrich, catalog number: P7626)
19. β-Glycerophosphate (β-GP) (Sigma-Aldrich, catalog number: G9422)
20. Sodium dihydrogen phosphate monohydrate (NaH2PO4·H2O) (Sigma-Aldrich, catalog number: S9638)
21. Disodium hydrogen phosphate heptahydrate (Na2HPO4·7H2O) (Sigma-Aldrich, catalog number: S9390)
22. Ethylenediaminetetraacetic acid disodium salt dihydrate (EDTA-Na2) (Sigma-Aldrich, catalog number: E4884)
23. Buffer solution 4.01 (Mettler Toledo, catalog number: 51350004)
24. Buffer solution 7.00 (Mettler Toledo, catalog number: 51350006)
25. Buffer solution 9.21 (Mettler Toledo, catalog number: 51350008)
26. 1,4-Dithiothreitol (DTT) (Roche, catalog number: 10197777001)
27. EZ-link alkoxyamine-PEG4-biotin (VWR, catalog number: PI26137)
28. p-Phenylenediamine (pPDA) (Sigma-Aldrich, catalog number: P6001)
29. Dimethyl sulfoxide (DMSO) (Sigma-Aldrich, catalog number: D8418)
30. Resolving gel buffer (Bio-Rad, catalog number: 1619798)
31. Stacking gel buffer (Bio-Rad, catalog number: 1619799)
32. 2-Mercaptoethanol (Carl Roth, catalog number: 4227.3)
33. Glycerol (Kemika, catalog number: 0711901)
34. 3-Color prestained protein marker (10–190 kDa) (MCE MedChemExpress, catalog number: HY-K1011), or equivalent prestained protein ladder
35. 30% Acrylamide-bis solution (Bio-Rad, catalog number: 1610156)
36. N,N,N’,N’-Tetramethyl ethylenediamine (TEMED) (Bio-Rad, catalog number: 161-0800)
37. Ammonium persulfate (APS) (Carl Roth, catalog number: 9178.3)
38. Methanol (Merck KGaA, catalog number: 1060121000)
39. Glacial acetic acid (Honeywell, catalog number: 33209)
40. InstantBlue Coomassie protein stain (Abcam, catalog number: ab119211)
41. Ponceau S (Thermo Scientific, catalog number: J60744.09)
42. Pierce ECL western blotting substrate (Thermo Scientific, catalog number: 32209)
43. Streptavidin, HRP conjugate (EMD Millipore, catalog number: 18-152)
44. Nonfat dry milk (Dukat)
45. Sodium chloride (NaCl) (Fisher Scientific, catalog number: S271500)
46. Bovine serum albumin (BSA) (Capricorn scientific, catalog number: BSA-1S)
47. Bromophenol blue (Sigma-Aldrich, catalog number: B0126)
48. Sodium hydroxide (NaOH) (Sigma-Aldrich, catalog number: S5881)
49. Hydrochloric acid (HCl) (Sigma-Aldrich, catalog number: 258148)
50. Isopropanol (Sigma-Aldrich, catalog number: 34863)
Solutions
1. Cell culture medium (see Recipes)
2. 0.1 M phosphate buffer, pH 7.4 (see Recipes)
3. 5 M NaCl (see Recipes)
4. RIPA phosphate lysis buffer with protease and phosphatase inhibitors (see Recipes)
5. 200 mM DTT (see Recipes)
6. 500 mM pPDA with 5 mM DTT (see Recipes)
7. 100 mM biotin-aminooxy (see Recipes)
8. 4× SDS-PAGE loading buffer (reducing) (see Recipes)
9. 10% Ammonium persulfate solution (see Recipes)
10. 10% SDS (see Recipes)
11. Resolving gel buffer (see Recipes)
12. Stacking gel buffer (see Recipes)
13. 10× SDS-PAGE running buffer (see Recipes)
14. 1× SDS-PAGE running buffer (see Recipes)
15. Western blotting transfer buffer (see Recipes)
16. Ponceau S staining solution (see Recipes)
17. 10× Tris-buffered saline, Tween-20 (TBS-T) (see Recipes)
18. Blocking solution (see Recipes)
19. 1:1,000 Streptavidin (HRP conjugate) solution (see Recipes)
20. ECL detection solution (see Recipes)
21. Resolving gel solution (see Recipes)
22. 4% stacking gel solution (see Recipes)
Recipes
1. Cell culture medium
| Reagent | Final concentration | Quantity or Volume | Quantity or Volume |
|---|---|---|---|
| FBS | 10% | 50 mL | 56.8 mL |
| L-glutamine | 1% | 5 mL | 5.7 mL |
| Penicillin-streptomycin | 1% | 5 mL | 5.7 mL |
| DMEM | n/a | 440 mL | 500 mL |
Keep at 4 °C.
2. 0.1 M phosphate buffer, pH 7.4
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Sodium dihydrogen phosphate | 0.1 M | 96.5 mL of 0.2 M stock solution |
| Disodium hydrogen phosphate | 0.1 M | 153.5 mL of 0.2 M stock solution |
| dH2O | n/a | 200 mL |
| 5 M HCl or 5 M NaOH | pH 7.4 | As needed to adjust pH to 7.4 |
| dH2O | n/a | Up to 500 mL |
dH2O: deionized H2O
Stock solutions
a. 0.2 M Sodium dihydrogen phosphate
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| NaH2PO4·H2O | 0.2 M | 27.598 g (MW: 137.99 g/mol) |
| dH2O | n/a | Up to 1 L |
Store at room temperature.
b. 0.2 M Disodium hydrogen phosphate
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Na2HPO4·7H2O | 0.2 M | 53.614 g (MW: 268.07 g/mol) |
| dH2O | n/a | Up to 1 L |
Store at room temperature.
3. 5 M NaCl
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| NaCl | 5 M | 146.1 g NaCl (MW: 58.44 g/mol) |
| dH2O | n/a | Up to 500 mL |
Store at room temperature.
4. RIPA phosphate lysis buffer with protease and phosphatase inhibitors
To the RIPA phosphate lysis buffer base, add protease and phosphatase inhibitors as indicated.
Protease/phosphatase inhibitors are prepared as stock solutions in distilled water, stored frozen, and added into the lysis buffer just prior to use as follows:
| Reagent | Stock solution concentration | Volume per 1 mL lysis buffer |
|---|---|---|
| Aprotinin | 1 mg/mL | 5 μL |
| Leupeptin | 10 mg/mL | 5 μL |
| Sodium fluoride (NaF) | 0.2 M | 5 μL |
| Sodium vanadate (Na3VO4) | 0.2 M | 5 μL |
| PMSF | 0.1 M | 15 μL |
| β-glycerophosphate | 2 M | 40 μL |
Alternatively, dissolve one tablet of Complete Mini EDTA-free protease inhibitor cocktail in 10 mL of phosphate RIPA lysis buffer base. Aliquot and keep at -20 °C.
Phosphate RIPA lysis buffer base (50 mL)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Phosphate buffer, pH 7.4 | 10 mM | 5 mL of 0.1 M stock solution |
| NaCl | 150 mM | 1.5 mL of 5 M NaCl |
| NP-40 | 1% | 0.714 g TERGITOLTM solution (70% NP-40 in H2O) |
| DTT | 5 mM | 38.575 mg DTT |
| Sodium deoxycholate | 0.1% | 50 mg |
| dH2O | n/a | Up to 50 mL |
The lysis buffer base is kept at 4 °C.
5. 200 mM DTT
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| DTT | 200 mM | 15.43 mg (MW: 154.3 g/mol) |
| dH20 | n/a | Up to 500 μL |
Prepare fresh just before use.
6. 500 mM pPDA with 5 mM DTT
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| pPDA | 500 mM | 55.17 mg pPDA (MW: 108.14 g/mol) |
| DMSO | n/a | 97.5 μL |
| DTT | 200 mM | 2.5 μL |
For easier preparation of the reagent needed for multiple labeling reactions, it is recommended to weigh a larger amount and then adjust the volume of DMSO accordingly. For instance, if 200 mg of pPDA is placed in a 1.5 mL Eppendorf tube, 353.45 μL of DMSO should be added and vortexed until fully dissolved. Subsequently, for each 97.5 μL of this solution, 2.5 μL of freshly prepared 200 mM DTT should be added to create the final solution.
7. 100 mM biotin-aminooxy
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| EZ-Link Alkoxyamine-PEG4-Biotin | 100 mM | 50.0 mg (MW: 154.3 g/mol) |
| DMSO | n/a | 1,151 μL |
Dissolve biotin-aminooxy in DMSO, make small, single-experiment aliquots, and store at -80 °C. Avoid multiple freeze-thaw cycles. This solution is stable for at least one year.
8. 4× SDS-PAGE loading buffer (reducing)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| 0.5 M Tris-HCl, pH 6.8 | 0.25 M | 5 mL |
| Glycerol | 40% (v/v) | 4 mL |
| SDS | 8% (w/v) | 0.8 g |
| 2-Mercaptoethanol (2-ME) | 4% (v/v) | 0.4 mL |
| Bromophenol blue | 0.01% | 4 mg |
| dH2O | n/a | Up to 10 mL |
9. 10% Ammonium persulfate solution
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Ammonium persulfate (APS) | 10% | 100.0 mg |
| dH2O | n/a | 1 mL |
Prepare fresh for each experiment.
10. 10% SDS
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Sodium dodecyl sulfate (SDS) | 10% | 10.0 g |
| dH2O | n/a | Up to 100 mL |
Store at room temperature.
11. Resolving gel buffer, 1.5 M Tris-HCl, pH 8.8
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Tris base | 1.5 M | 181.7 g |
| dH2O | n/a | Up to 800 mL |
| HCl (37%) | pH 8.8 | As needed to adjust pH to pH 8.8 |
| dH2O | n/a | Up to 1 L |
Store at 4 °C.
12. Stacking gel buffer, 0.5 M Tris-HCl, pH 6.8
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Tris base | 0.5 M | 60.6 g |
| dH2O | n/a | Up to 800 mL |
| HCl (37%) | pH 6.8 | As needed to adjust pH to pH 6.8 |
| dH2O | n/a | Up to 1 L |
Store at 4 °C.
13. 10× SDS-PAGE running buffer
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Tris base | 0.25 M | 30.29 g (MW: 121.14 g/mol) |
| Glycine | 1.92 M | 144.13 g (MW: 75.07 g/mol) |
| SDS | 0.1% | 10 mL from 10% SDS |
| dH2O | n/a | 1 L |
Dissolve in 900 mL of dH2O, adjust to 1 L with dH2O, and store at room temperature. The buffer has a pH of around 8.3. Do not adjust its pH.
14. 1× SDS-PAGE running buffer
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| 10× SDS-PAGE running buffer | 1× | 100 mL |
| SDS | 0.1% | 10 mL of 10% SDS solution |
| dH2O | n/a | Up to 1 L |
Keep at room temperature. If used infrequently, store at 4 °C.
15. Western blotting transfer buffer
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Tris base | 25 mM | 3.03 g (MW: 121,14 g/mol) |
| Glycine | 0.192 M | 14.4 g (MW: 75.07 g/mol) |
| Methanol | 20% | 200 mL |
| dH2O | n/a | Up to 1 L |
Prepare on the day of transfer and cool down at -20 °C while running the SDS-PAGE.
16. Ponceau S staining solution
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Ponceau S | 0.1% (wt/vol) | 0.05 g |
| Glacial acetic acid | 5% (v/v) | 2.5 mL |
| dH2O | n/a | Up to 50 mL |
Add ultrapure water up to 50 mL, wrap in aluminum foil, and store at room temperature.
17. 10× Tris-buffered saline, Tween-20 (TBS-T)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Tris base | 50 mM | 6.05 g (MW: 121.14 g/mol) |
| NaCl | 150 mM | 8.76 g (MW: 58.44 g/mol) |
| HCl (37%) | pH 7.5 | Adjust to pH 7.5 |
| Tween-20 | 0.1 % | 1 mL |
| dH2O | n/a | Up to 1 L |
Add ultrapure water up to 800 mL and set the pH value up to 7.5. Add 1 mL of Tween-20 and add water up to 1,000 mL. Store at 4 °C.
18. Blocking solution
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Nonfat dry milk | 5% | 2.5 g |
| 10× TBS-T | 1× | 5 mL |
| dH2O | n/a | Up to 50 mL |
Prepare on the day of transfer and keep at 4 °C.
19. 1:1,000 Streptavidin-HRP conjugate solution
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Streptavidin-HRP | 1‰ | 10 μL |
| 10× TBS-T | 1× | 1 mL |
| dH2O | n/a | Up to 10 mL |
Mix well. Prepare just prior to the incubation with the membrane.
20. ECL detection solution
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Pierce ECL Solution A | 50% | 2.0 mL |
| Pierce ECL Solution B | 50% | 2.0 mL |
Mix the solutions just before use. For a typical size of the membrane in Mini-Protean Tetra Cell (9 × 7 cm), 4 mL of ECL detection solution is sufficient.
21. Resolving gel solution
| Gel density | Milli-Q water (mL) | 30% acrylamide/ bis acrylamide (mL) | Resolving gel buffer (mL) | 10% SDS (μL) |
|---|---|---|---|---|
| 7% | 5.1 | 2.3 | 2.5 | 100 |
| 8% | 4.7 | 2.7 | 2.5 | 100 |
| 9% | 4.4 | 3.0 | 2.5 | 100 |
| 10% | 4.1 | 3.3 | 2.5 | 100 |
| 11% | 3.7 | 3.7 | 2.5 | 100 |
| 12% | 3.4 | 4.0 | 2.5 | 100 |
Add 50 µL of 10% APC and 5 µL of TEMED to each 10 mL of the resolving gel solution. Gently mix, then pour the mixture into the gel assembly.
22. 4% stacking gel solution
| Gel density | Milli-Q water (mL) | 30% acrylamide/ bis acrylamide (mL) | Stacking gel buffer (mL) | 10% SDS (μL) |
|---|---|---|---|---|
| 4% | 6.1 | 1.3 | 2.5 | 100 |
Add 50 µL of 10% APC and 5 µL of TEMED to each 10 mL of the stacking gel solution. Gently mix, then pour the mixture into the gel assembly.
Laboratory supplies
1. Parafilm® M laboratory film (Amcor, catalog number: PM-996)
2. 0.1–10 μL pipette tips (Eppendorf, catalog number: 0030000811)
3. 0.5–20 μL pipette tips (Eppendorf, catalog number: 0030000854)
4. 5–200 μL pipette tips (Eppendorf, catalog number: 0030000889)
5. 50–1,000 μL pipette tips (Eppendorf, catalog number: 0030000927)
6. 1.5 mL tubes (Eppendorf, catalog number: 0030120086)
7. 2.0 mL tubes (Eppendorf, catalog number: 0030120094)
8. 15 mL tubes (Sarstedt AG & Co. KG, catalog number: 62.554.502)
9. 50 mL tubes (Greiner bio-one, catalog number: 227261)
10. 2 mL serological pipettes (Falcon, catalog number: 357507)
11. 5 mL serological pipettes (Falcon, catalog number: 357543)
12. 10 mL serological pipettes (Falcon, catalog number: 357551)
13. T75 cell culture flasks (Sarstedt AG & Co. KG, catalog number: 83.3911.002)
14. Mini Trans-Blot® filter paper (Bio-Rad, catalog number: 1703932)
15. Immun-Blot® LF PVDF membrane roll (Bio-Rad, catalog number: 162-0264)
Equipment
1. Laminar air flow cabinet (Nüve, catalog number: LN090)
2. Cell incubator (Nüve, catalog number: EC160)
3. Inverted microscope (Olympus, model: CKX41)
4. Refrigerated laboratory centrifuge (Eppendorf, catalog number: 5920R)
5. Power supply (Bio-Rad, catalog number: 1645050)
6. Mini-PROTEAN 3 Cell (Bio-Rad, catalog numbers: 165-3301, 165-3302)
7. Mini trans-blot electrophoretic transfer cell (Bio-Rad, catalog number: 1703930)
8. Gel releaser for Mini-PROTEAN 3 (Bio-Rad, catalog number: 1653320)
9. Refrigerated microcentrifuge (Eppendorf, catalog number: 5427R)
10. 0.5–10 μL pipette (Eppendorf Research plus, catalog number: 3123000020)
11. 2–20 μL pipette (Eppendorf Research plus, catalog number: 3123000039)
12. 20–200 μL pipette (Eppendorf Research plus, catalog number: 3123000055)
13. 100–1,000 μL pipette (Eppendorf Research plus, catalog number: 3123000063)
14. Pipette filler (MediLab Tech, catalog number: 7013100200)
15. Eppendorf ThermoMixer® C (Eppendorf, catalog number: 5382000023)
16. 3D shaker (Cleaver Scientific, catalog number: MW-23)
17. Vortex shaker (IKA, catalog number: 10142902)
18. Laboratory balance (Radweg, catalog number: PS750.R1)
19. pH meter (HACH, catalog number: HQ30D53000000)
20. BioDropTM μLite+ (VWR, catalog number: 80-3006-55)
21. ChemiDocTM MP imaging system (Bio-Rad, catalog number: 12003154)
Software and datasets
1. Image Lab software (Bio-Rad)
Procedure
文章信息
稿件历史记录
提交日期: May 6, 2025
接收日期: Jun 16, 2025
在线发布日期: Jul 16, 2025
出版日期: Aug 5, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/).
如何引用
Mikulić, F. L., Merćep, V., Finek, M. and Merćep, M. (2025). Reliable and Sensitive Detection of Carbonylated Proteins by Oxime Blot. Bio-protocol 15(15): e5401. DOI: 10.21769/BioProtoc.5401.
分类
生物化学 > 蛋白质 > 电泳
细胞生物学 > 细胞新陈代谢 > 其它化合物
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link