- EN - English
- CN - 中文
Prokaryotic Expression and Purification of the hSox2-HMG Domain
hSox2-HMG结构域的原核表达与纯化
(*contributed equally to this work) 发布: 2025年08月20日第15卷第16期 DOI: 10.21769/BioProtoc.5394 浏览次数: 2362
评审: Sreesankar EaswaranVivek GurungAlba Blesa
Abstract
The Sox (SRY-related HMG-box) protein family plays a crucial role in cellular differentiation, development, and gene regulation, with the HMG (high-mobility group) domain responsible for DNA binding and transcriptional regulation. Proteins in the SOX gene family contain an HMG domain that shares 50% homology with the HMG domain of the sex-determining factor SRY gene. The SOX gene family comprises 30 proteins, which are classified into 10 groups (A–H). As a member of this family, hSox2 has been shown to be involved in various biological processes, but its specific function remains unclear. Previous studies have used eukaryotic expression systems, GST-tag purification, and bacterial inclusion body refolding techniques to produce Sox family proteins. However, these methods are often limited by issues such as low yield, incorrect folding, or inefficient purification, restricting their application in functional and structural studies. In this study, a prokaryotic expression system for the hSox2-HMG domain was constructed using the pET22b vector and Escherichia coli BL21(DE3) as the host strain. Protein expression was induced by IPTG, and initial purification was performed using Ni-NTA affinity chromatography, followed by ultrafiltration concentration and size exclusion chromatography to improve purity. By optimizing lysis and elution conditions, we successfully obtained hSox2-HMG protein with high expression levels and purity. This method provides a cost-effective and scalable strategy for hSox2-HMG production, ensuring high purity and correct folding of the protein. The optimized experimental protocol lays a foundation for structural and functional studies of hSox2-HMG.
Key features
• The hSox2-HMG protein was expressed in Escherichia coli BL21(DE3) using the pET22b vector and IPTG induction, resulting in high-yield recombinant protein.
• Ni-NTA affinity chromatography was employed for protein purification and combined with ultrafiltration concentration and size exclusion chromatography to enhance purity and ensure correct folding.
• The established workflow provides an efficient, cost-effective, and scalable strategy for hSox2-HMG production, suitable for structural and functional studies.
• The purified hSox2-HMG protein maintains structural integrity and can be used for further investigation of DNA-binding properties and regulatory functions.
Keywords: hSox2-HMG (hSox2-HMG)Graphical overview
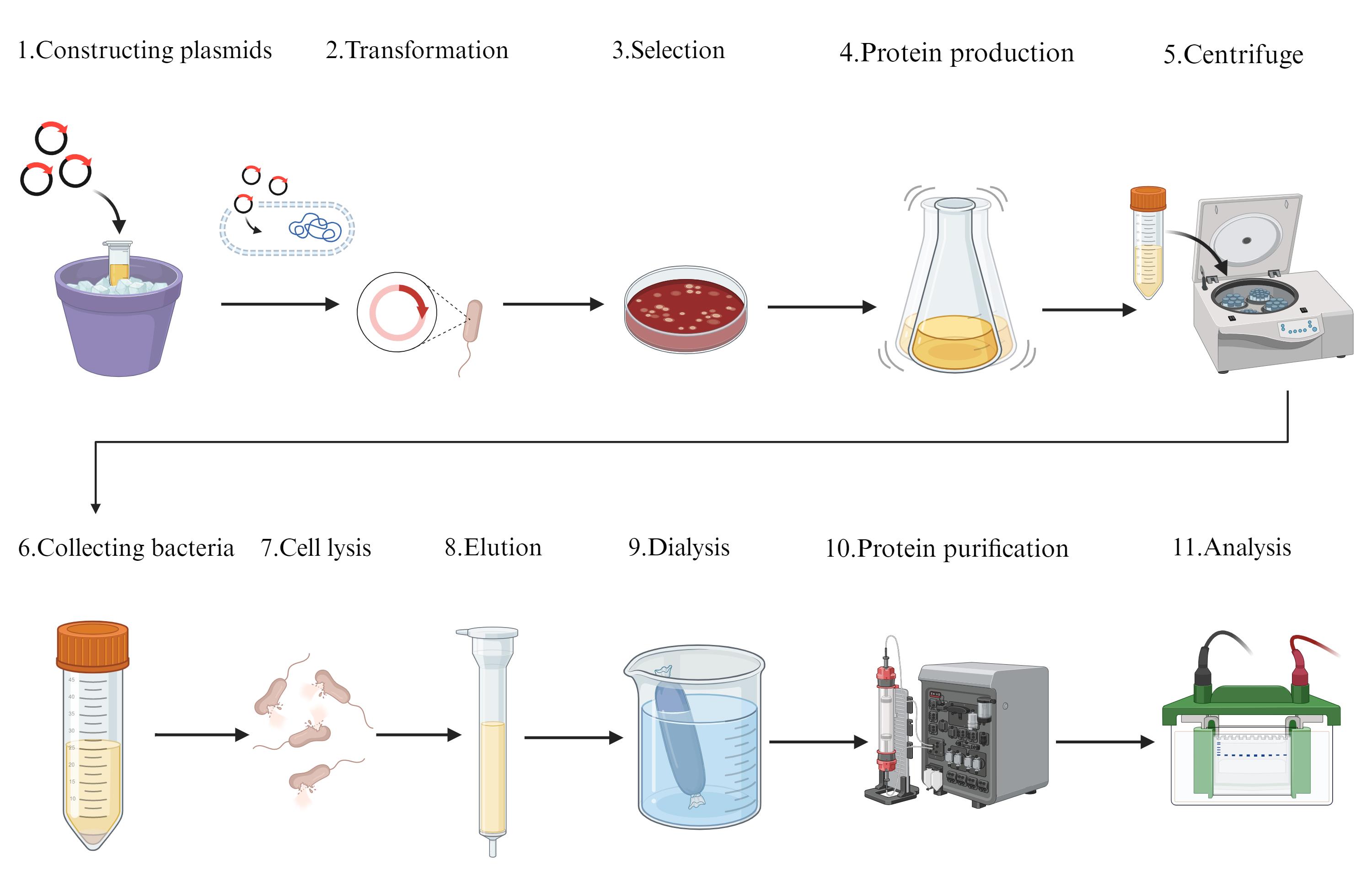
Flowchart of prokaryotic expression and purification of the hSOX2-HMG domain. Constructing plasmids: synthesized by General Biol (Anhui) Co. Transformation: E. coli BL21(DE3) cells and the expression plasmid (hSOX2-HMG-pET22b) were extracted on ice. Selection: all colonies on the LB plate were scraped for protein expression. Protein production: protein expression was performed using shake-flask culture. Centrifuge: cells were separated from the culture medium by centrifugation. Collecting bacteria: discard the culture medium. Cell lysis: cells were disrupted by sonication with an ultrasonic cell disruptor. Elution: through Ni-NTA. Dialysis: replacement buffer. Protein purification: through size exclusion. Analysis: through SDS-PAGE.
Background
In the regulation of embryogenesis, multiple transcription factors and proteins interacting with DNA play a crucial role, among which is the SOX2 within the SOX B1 subfamily. The SOX gene family is classified into 10 groups (A–H) [1]. The high-mobility group HMG-box (HMG) domain serves as the primary structural basis for DNA binding in SOX family transcription factors and is highly conserved [2]. The pioneer transcription factor OCT4 binds to the octameric motif (5′-ATTTGCAT-3′) and forms a trimeric complex with SOX2 or SOX15 on DNA, regulating the expression of genes involved in embryonic development, such as YES1, FGF4, UTF1, and ZFP206. It is crucial for early embryogenesis and the pluripotency of embryonic stem cells (ESCs) [3]. Shinya Yamanaka first demonstrated in 2007 that SOX2, in combination with three other transcription factors (Oct4/POU5F1, Klf4, and c-Myc/MYC), could cooperatively reprogram mouse fibroblast cells into induced pluripotent stem cells (iPSCs) through somatic cell reprogramming in vitro. When combined, these factors are sufficient to reprogram differentiated cells into an embryonic-like state, generating induced pluripotent stem (iPS) cells. iPS cells exhibit the morphology and growth characteristics of ESCs and express ESC marker genes [3]. SOX2 has emerged as the most extensively investigated transcription factor within the SOX B1 subfamily. However, conventional eukaryotic expression systems for the production of SOX2 are typically characterized by low yields, high production costs [4], and a requirement for expensive affinity chromatography media during purification. In contrast, the present study introduces an efficient prokaryotic alternative. Utilizing Escherichia coli as the expression host, we achieved predominantly soluble expression of SOX2 HMG, thereby circumventing the necessity for the inclusion of body refolding. The purification protocol delineated herein consistently affords high-purity SOX2 HMG protein, establishing a robust and cost-effective methodology for its production.
Materials and reagents
Biological materials
Strains
1. Escherichia coli BL21 (DE3) competent cells (Sangon Biotech, catalog number: B528414-0100)
Plasmids
The codon-optimized cDNA sequence (see specific sequences below) for hSOX2 HMG domain was synthesized by General Biol (Anhui) Co. and was subsequently cloned into the pET22b (+) expression vector [from General Biol (Anhui) Co., sequence verified] with Nde I and Xho I cloning sites, to produce hSOX2-HMG-6×His-pET22b expression plasmid.
Codon-optimized cDNA base sequence:
CATATGGATCGTGTGAAACGTCCGATGAATGCATTCATGGTGTGGAGCCGTGGTCAGCGCCGCAAAATGGCACAGGAAAATCCGAAAATGCATAATAGTGAAATCAGTAAACGCCTGGGTGCAGAATGGAAACTGCTGAGCGAAACCGAAAAACGTCCGTTTATTGATGAAGCAAAACGCCTGCGCGCCCTGCACATGAAAGAACATCCGGATTATAAATATCGCCCGCGTCGCAAAACCAAAACCCTCGAG
Underlined are the hSOX2-HMG DNA base sequences.
SOX2 binding DNA sequence:
P1: 5′-GGAAACAATGGA-3′ (transcription factor SOX2 binds to DNA at the 5′-AACAATG-3′ consensus sequence)
P2: 5′-TCCATTGTTTCC-3′
The DNA base sequence recognized by hSOX2 was synthesized by Sangon Biotech. Double-stranded DNA was prepared through annealing.
Reagents
1. Tryptone (Sangon Biotech, CAS No.: 73049-73-7)
2. Yeast extract (Sangon Biotech, CAS No.: 8013-01-2)
3. NaCl (MACKLIN, CAS No.: 7647-14-5)
4. Agarose (Biowest, CAS No.: 9012-36-6)
5. Sodium phosphate dibasic (Na2HPO4) (MACKLIN, CAS No.: 7558-79-4)
6. Potassium phosphate monobasic (KH2PO4) (MACKLIN, CAS No.: 7778-77-0)
7. Ampicillin sodium (Amp) (BBI, CAS No.: 69-52-3)
8. Isopropyl-β-D-thiogalactopyranoside (IPTG) (Aladdin, CAS No.: 367-93-1)
9. Ethylenediaminetetraacetic acid (EDTA) (Aladdin, CAS No.: 60-00-4)
10. Ni-NTA agarose (Qiagen, catalog number: 30230)
11. Glycerol (Aladdin, CAS No.: 56-81-5)
12. Phenylmethylsulfonyl fluoride (PMSF) (Aladdin, CAS No.: 329-98-6)
13. Urea (MACKLIN, CAS No.: 57-13-6)
14. Imidazole (MACKLIN, CAS No.: 288-32-4)
15. Sodium dodecyl sulfate (SDS) (BioFroxx, CAS No.: 151-21-3)
16. Ammonium persulfate (APS) (Aladdin, CAS No.: 7727-54-0)
17. Hydroxymethyl aminomethane (Tris) (MACKLIN, CAS No.: 77-86-1)
18. Tetramethylethylenediamine (TEMED) (Aladdin, CAS No.: 110-18-9)
19. Acrylamide (Sangon Biotech, CAS No.: 79-06-1)
20. N,N'-Methylenebisacrylamide (MACKLIN, CAS No.: 110-26-9)
21. Coomassie brilliant blue G-250 (BBI, CAS No.: 6104-58-1)
22. Isopropyl alcohol (Aladdin, CAS No.: 67-63-0)
23. Acetic acid (Sangon Biotech, CAS No.: 64-19-17)
24. β-mercaptoethanol (β-ME) (Aladdin, CAS No.: 60-24-2)
25. NaOH (BBI, CAS No.: 1310-73-2)
26. Boric acid (MACKLIN, CAS No.: 10043-35-3)
27. Glycine (Meilunbio, CAS No.: 56-40-6)
28. Prestained protein ladder (10–180 kDa) (SmartBuffers, catalog number: N6616)
29. Bromophenol blue (Aladdin, CAS No.: 115-39-9)
30. Enhanced BCA Protein Assay kit (Biosharp, catalog number: BL1054C)
31. Glycine (MACKLIN, CAS No.: 50-01-1)
32. HCl (Sinopharm Chemical Reagent Co., Ltd., CAS No.: 7647-01-0)
33. DL-Dithiothreitol (Aladdin, CAS No.: 3483-12-3)
34. Agar (Sangon Biotech, CAS No.: 9002-18-0)
Solutions
1. LB liquid/agar solid medium (see Recipes)
2. Ni affinity chromatography purification binding buffer (washing buffer 1) (see Recipes)
3. Ni affinity chromatography purification washing buffer 2 (see Recipes)
4. Ni affinity chromatography purification washing buffer 3 (see Recipes)
5. Ni affinity chromatography purification elution buffer 1 (see Recipes)
6. Ni affinity chromatography purification elution buffer 2 (see Recipes)
7. 6% PAGE (see Recipes)
8. 15% SDS-PAGE (see Recipes)
9. 50% (v/v) glycerol (see Recipes)
10. Ampicillin stock (see Recipes)
11. 0.5 M IPTG (see Recipes)
12. Gel filtration buffer (see Recipes)
13. Coomassie brilliant blue stain (see Recipes)
14. 5× TBE (see Recipes)
15. 0.5 M EDTA pH 8.0 (see Recipes)
16. 5× Running buffer (see Recipes)
17. 5× SDS loading buffer (see Recipes)
18. 6 M Guanidine (see Recipes)
19. 5 M imidazole pH 7.2 or 8.0 (see Recipes)
20. 30% Acrylamide (see Recipes)
21. 1.5 M Tris-HCl pH 8.8 (see Recipes)
22. 1.0 M Tris-HCl pH 6.8 (see Recipes)
23. 10% SDS (see Recipes)
24. 10% APS (see Recipes)
25. 1 M DL-Dithiothreitol (DTT) (see Recipes)
Recipes
1. LB liquid/agar solid medium (100 mL)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Tryptone | 7 g/L | 0.7 g |
| Yeast extract | 3.5 g/L | 0.35 g |
| Sodium chloride | 10 g/L | 1 g |
| Agar powder (alternative) | 20 g/L | 2 g |
| H2O | n/a | 100 mL |
Prepare fresh and use immediately. Make up the volume to 1,00 mL. After mixing all reagents thoroughly, sterilize by autoclaving on liquid setting (121 °C for 20 min).
2. Ni affinity chromatography purification binding buffer (washing buffer 1) (1,000 mL)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Imidazole pH 8.0 | 10 mM | 2 mL |
| NaCl | 0.5 M | 125 mL |
| Tris-HCl pH 8.0 | 20 mM | 20 mL |
| Glycerol | 0.2% | 4 mL |
| β-ME | 1 mM | 1 mL |
| Urea | 0.1 M | 14 mL |
| H2O | n/a | To 1,000 mL |
Use a preconfigured solution: 5 M imidazole pH 8.0, 4 M NaCl, 1 M Tris-HCl pH 8.0, 50% glycerol, 1 M β-ME, and 7 M Urea. Add ultrapure water to a final volume of 1,000 mL. Store at 4 °C for up to four weeks.
3. Ni affinity chromatography purification washing buffer 2 (1,000 mL)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Imidazole pH 8.0 | 25 mM | 5 mL |
| NaCl | 0.5 M | 125 mL |
| Tris-HCl pH 8.0 | 20 mM | 20 mL |
| Glycerol | 0.2% | 4 mL |
| β-ME | 1 mM | 1 mL |
| Urea | 0.1 M | 14 mL |
| H2O | n/a | To 1,000 mL |
Use a preconfigured solution: 5 M imidazole pH 8.0, 4 M NaCl, 1 M Tris-HCl pH 8.0, 50% glycerol, 1 M β-ME, and 7 M Urea. Add ultrapure water to a final volume of 1,000 mL. Store at 4 °C for up to four weeks.
4. Ni affinity chromatography purification washing buffer 3 (1,000 mL)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Imidazole pH 8.0 | 50 mM | 10 mL |
| NaCl | 0.5 M | 125 mL |
| Tris-HCl pH 8.0 | 20 mM | 20 mL |
| Glycerol | 0.2% | 4 mL |
| β-ME | 1 mM | 1 mL |
| Urea | 0.1 M | 14 mL |
| H2O | n/a | To 1,000 mL |
Use a preconfigured solution: 5 M imidazole pH 8.0, 4 M NaCl, 1 M Tris-HCl pH 8.0, 50% glycerol, 1 M β-ME, and 7 M Urea. Add ultrapure water to a final volume of 1,000 mL. Store at 4 °C for up to four weeks.
5. Ni affinity chromatography purification elution buffer 1 (1,000 mL)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Imidazole pH 7.2 | 250 mM | 50 mL |
| NaCl | 0.5 M | 125 mL |
| Tris-HCl pH 7.2 | 20 mM | 20 mL |
| Glycerol | 0.2% | 4 mL |
| β-ME | 1 mM | 1 mL |
| Urea | 0.1 M | 14 mL |
| H2O | n/a | To 1,000 mL |
Use a preconfigured solution: 5 M imidazole pH 7.2, 4 M NaCl, 1 M Tris-HCl pH 7.2, 50% glycerol, 1 M β-ME, and 7 M Urea. Add ultrapure water to a final volume of 1,000 mL. Store at 4 °C for up to four weeks.
6. Ni affinity chromatography purification elution buffer 2 (1,000 mL)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Imidazole pH 7.2 | 500 mM | 100 mL |
| NaCl | 0.5 M | 125 mL |
| Tris-HCl pH 7.2 | 20 mM | 20 mL |
| Glycerol | 0.2% | 4 mL |
| β-ME | 1 mM | 1 mL |
| Urea | 0.1 M | 14 mL |
| H2O | n/a | To 1,000 mL |
Use a preconfigured solution: 5 M imidazole pH 7.2, 4 M NaCl, 1 M Tris-HCl pH 7.2, 50% glycerol, 1 M β-ME, and 7 M Urea. Add ultrapure water to a final volume of 1,000 mL. Store at 4 °C for up to four weeks.
7. 6% PAGE (16 mL)
| Reagent | Volume |
|---|---|
| 5× TBE | 1.6 mL |
| 30% Acrylamide | 3.2 mL |
| 10% APS | 150 μL |
| TEMED | 20 μL |
| H2O | To 16 mL |
Prepare fresh and use immediately. Add 5× TBE, 30% Acrylamide, 10% APS, TEMED, and H2O in that order. Insert a 15-well 1 mm comb and let it solidify at room temperature for about 2 h.
8. 15% SDS-PAGE
| Reagent | Volume (15 mL) Separation gel | Reagent | Volume (6 mL) Stacking gel |
|---|---|---|---|
| ddH2O | 3.4 mL | ddH2O | 4.2 mL |
| 30% Acrylamide | 7.5 mL | 30% Acrylamide | 1 mL |
| 1.5 M Tris-HCl pH 8.8 | 3.8 mL | 1.0 M Tris-HCl pH 6.8 | 760 μL |
| 10% SDS | 150 μL | 10% SDS | 60 μL |
| 10% APS | 150 μL | 10% APS | 60 μL |
| TEMED | 6 μL | TEMED | 3 μL |
Prepare fresh and use immediately. Add ddH2O, 30% Acrylamide, 1.0 M Tris-HCl pH 6.8 (1.5 M Tris-HCl pH 8.8), 10% SDS, 10% APS, and TEMED in that order. Insert a 15-well 1 mm comb and let it solidify at room temperature for about 1 h.
9. 50% (v/v) glycerol (100 mL)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| 100% (v/v) glycerol | 50% (v/v) | 50 mL |
| H2O | n/a | 50 mL |
Mix 100% (v/v) glycerol and H2O (pour pure glycerol into the H2O). Stir for 5 min and then transfer to a 100 mL bottle. Sterilize by autoclaving on liquid setting (121 °C for 20 min). Store at 4 °C for up to four weeks.
10. Ampicillin stock (10 mL)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Ampicillin | 100 g/L | 1 g |
| H2O | n/a | 10 mL |
Sterilize by filtering with a 0.2 μm syringe-end filter. Store in 1 mL aliquots at -20 °C indefinitely.
11. 0.5 M IPTG (10 mL)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| IPTG | 0.5 M | 1.19 g |
| H2O | n/a | 10 mL |
Sterilize by filtering with a 0.2 μm syringe-end filter. Store in 1 mL aliquots at -20 °C indefinitely.
12. Gel filtration buffer (1,000 mL)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| NaCl | 0.5 M | 125 mL |
| Tris-HCl pH 7.2 | 20 mM | 20 mL |
| EDTA | 1 mM | 2 mL |
| H2O | n/a | To 1,000 mL |
Prepare fresh and use immediately. Use a preconfigured solution: 4 M NaCl, 1 M Tris-HCl pH 7.2, and 0.5 M EDTA. Add ultrapure water to a final volume of 1,000 mL.
13. Coomassie brilliant blue stain
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Coomassie brilliant blue G-250 | 0.5 g/L | 0.5 g |
| Isopropyl alcohol | 25% (v/v) | 250 mL |
| Acetic acid | 10% (v/v) | 100 mL |
| H2O | n/a | To 1,000 mL |
Mix 0.5 g of Coomassie brilliant blue G-250, isopropyl alcohol, and acetic acid, and wait until Coomassie brilliant blue G-250 is completely dissolved. Add ultrapure water to a final volume of 1,000 mL. Store at room temperature for up to four weeks.
14. 5× TBE (1,000 mL)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Tris | 54 g/L | 54 g |
| Boric acid | 27.5 g/L | 27.5 g |
| 0.5 M EDTA pH 8.0 | 10 mM | 20 mL |
| H2O | n/a | To 1,000 mL |
Add ultrapure water to a final volume of 1,000 mL. Mix well and filter using a 0.22-μm filter. Store at room temperature for up to four weeks.
15. 0.5 M EDTA pH 8.0 (1,000 mL)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| EDTA | 0.5 M | 146.12 g |
| H2O | n/a | To 1,000 mL |
Add solid NaOH and adjust pH to 8.0. Add ultrapure water to a final volume of 1,000 mL. Mix well and filter using a 0.22-μm filter. Store at 4 °C for up to four weeks.
16. 5× running buffer (1,000 mL)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Tris | 15 g/L | 15 g |
| SDS | 5 g/L | 5 g |
| Glycine | 72 g/L | 72 g |
| H2O | n/a | To 1,000 mL |
Add ultrapure water to a final volume of 1,000 mL. Mix well and filter using a 0.22-μm filter. The working concentration was diluted to 1×. Store at room temperature for up to four weeks.
17. 5× SDS loading buffer (100 mL)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| 1 M Tris-HCl pH 6.8 | 0.25 M | 25 mL |
| 10% SDS (w/v) | 2% (w/v) | 20 mL |
| 50% Glycerol (v/v) | 25% (w/v) | 50 mL |
| 1 M DTT | 20 mM | 2 mL |
| Bromophenol blue | 0.2 g/L | 0.02 g |
| H2O | n/a | To 100 mL |
Add ultrapure water to a final volume of 100 mL. Store indefinitely at -20 °C.
18. 6 M Guanidine (1,000 mL)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Guanidine | 6 M | 573.18 g |
| H2O | n/a | To 1000 mL |
Add ultrapure water to a final volume of 1,000 mL. Mix well and filter using a 0.22-μm filter. Store at room temperature for up to four weeks.
19. 5 M imidazole pH 7.2 or 8.0 (500 mL)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Imidazole | 5 M | 170.20 g |
| H2O | n/a | To 500 mL |
Add HCl and adjust pH to 7.2 or 8.0. Add ultrapure water to a final volume of 500 mL. Mix well and filter using a 0.22-μm filter. Store at 4 °C for up to four weeks.
20. 30% Acrylamide (1,000 mL)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Acrylamide | 292 g/L | 292 g |
| N,N'-Methylenebisacrylamide | 8 g/L | 8 g |
| H2O | n/a | To 1,000 mL |
Add ultrapure water to a final volume of 1,000 mL. Mix well and store at 4 °C for up to four weeks.
21. 1.5 M Tris-HCl pH 8.8 (1,000 mL)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Tris | 1.5 M | 181.71 g |
| H2O | n/a | To 1,000 mL |
Add HCl and adjust pH to 8.8. Add ultrapure water to a final volume of 1,000 mL. Mix well and filter using a 0.22-μm filter. Store at room temperature for up to four weeks.
22. 1.0 M Tris-HCl pH 6.8 (1,000 mL)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Tris | 1 M | 121.14 g |
| H2O | n/a | To 1,000 mL |
Add HCl and adjust pH to 6.8. Add ultrapure water to a final volume of 1,000 mL. Mix well and filter using a 0.22-μm filter. Store at room temperature for up to four weeks.
23. 10% SDS (1,000 mL)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| SDS | 100 g/L | 100 g |
| H2O | n/a | To 1,000 mL |
Add ultrapure water to a final volume of 1,000 mL. Mix well and store at room temperature for up to four weeks.
24. 10% APS (50 mL)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| APS | 100 g/L | 5 g |
| H2O | n/a | To 50 mL |
Add ultrapure water to a final volume of 50 mL. Mix well and store at 4 °C for up to four weeks.
25. 1 M DL-Dithiothreitol (DTT) (50 mL)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| DL-Dithiothreitol | 1 M | 7.71 g |
| H2O | n/a | To 50 mL |
Add ultrapure water to a final volume of 50 mL. Mix well and store in 1 mL aliquots at -20 °C indefinitely.
Laboratory supplies
1. 60 mL gravity column (Beyotime, catalog number: FCL60)
2. 3 K dialysis tubes (Solarbio, catalog number: YA1078)
3. 3 KDa concentration tubes (Millipore, catalog number: UFC9030)
Equipment
1. Autoclave (Shanghai Boxun Medical Biological Instrument Corp., model: YXQ-50SII)
2. Temperature-controlled incubator (Mingtu Machinery Equipment Co., model: SPX-2508)
3. Shaker incubator (Shanghai Zhichu Instrument Co., Ltd., model: ZQZY-AF8)
4. Spectrophotometer (Yipu Instrument Manufacturing Co., Ltd., model: U-T6)
5. Normal temperature, low-speed centrifuge (Xicheng Xinrui Instrument Plant, Jintan District, model: TDL-5A)
6. Freezer (-80 °C) (Thermo Fisher Scientific, model: 902ULTS)
7. Ultrasonic cell disruptor [Gallop Instrument (Shanghai) Co., Ltd, model: UCD-650B]
8. High-speed refrigerated centrifuge (Hunan Kecheng Instrument & Equipment Co., Ltd., model: W3-16R)
9. Low-speed refrigerated centrifuge (Hunan Kecheng Instrument & Equipment Co., Ltd., model: L4-5KR)
10. Electrophoresis apparatus (Shanghai Tanon Life Science Co., Ltd., model: VE-80)
11. Gel imaging system (MIULAB, model: GIS-630)
12. Single-wavelength protein purification system (GE Healthcare, model: ÄKTA prime Plus)
13. Micro high-speed refrigerated centrifuge [Gallop Instrument (Shanghai) Co., Ltd, model: MH-16KR]
Software and datasets
1. GraphPad Prism (for analysis and drawing of size exclusion chromatography)
2. ImageJ (for analysis of electrophoretic mobility shift assay)
Procedure
文章信息
稿件历史记录
提交日期: Apr 10, 2025
接收日期: Jun 22, 2025
在线发布日期: Aug 7, 2025
出版日期: Aug 20, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Yang, L., Tan, W. and Hong, J. (2025). Prokaryotic Expression and Purification of the hSox2-HMG Domain. Bio-protocol 15(16): e5394. DOI: 10.21769/BioProtoc.5394.
分类
生物化学 > 蛋白质 > 表达
生物化学 > 蛋白质 > 分离和纯化
微生物学 > 异源表达系统 > 大肠杆菌
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link