- EN - English
- CN - 中文
Two-photon (2P) Microscopy to Study Ca2+ Signaling in Astrocytes From Acute Brain Slices
利用双光子显微镜研究急性脑片中星形胶质细胞的钙信号
发布: 2025年07月05日第15卷第13期 DOI: 10.21769/BioProtoc.5371 浏览次数: 2867
评审: Achira RoyAbhishek VatsAnonymous reviewer(s)

相关实验方案
利用基于 FRET 的 SuperClomeleon 传感器监测器官型海马切片中细胞内氯离子水平变化
Sam de Kater [...] Corette J. Wierenga
2025年03月05日 2780 阅读
Abstract
Since the discovery that astrocytes are characterized by Ca2+-based excitability, investigating the function of these glial cells within the brain requires Ca2+ imaging approaches. The technical evolution from chemical fluorescent Ca2+ probes with low cellular specificity to genetically encoded indicators (GECIs) has enabled detailed analysis of the spatial and temporal features of intracellular Ca2+ signal. Different imaging methodologies allow the extraction of distinct information on calcium signals in astrocytes from brain slices, with resolution ranging from cell populations to single cells up to subcellular domains.
Here, we describe 2-photon laser scanning microscopy (2PLSM) Ca2+ imaging in astrocytes from the somatosensory cortex (SSCx) of adult mice in ex vivo acute cortical slices, performed using two genetically encoded Ca2+ indicators, i.e., cytosolic GCaMP6f and endoplasmic reticulum-targeted G-CEPIA1er. The main advantage of the 2PLSM technique, compared to single-photon microscopy, is the possibility to go deeper in the tissue while avoiding photodamage, by limiting laser excitation to a single focal plane. The fluorescent signal of the indicator is analyzed offline in different compartments—soma, proximal processes, and microdomains—for GCaMP6f experiments and in the perinuclear, somatic area for G-CEPIA1er. The analysis of Ca2+ signal from different compartments, although not providing a value of absolute concentration, allows a critical comparison of the degree of astrocyte activation between different experimental conditions or mouse models. Moreover, the analysis of G-CEPIA1er signal, which reveals metabotropic receptor activation as a dynamic decrease in free Ca2+ in the endoplasmic reticulum (ER), can provide information on possible alterations in this critical second messenger pathway in astrocytes, including, for example, steady-state ER Ca2+ levels and kinetics of Ca2+ release.
Key features
• This protocol is useful to characterize basal and evoked Ca2+ astrocyte activity in acute mouse brain slices, deepening analysis to different subcellular territories and compartments.
• The induction of Ca2+ probe expression requires surgical experience in mice and appropriate stereotaxic equipment for adeno-associated viral (AAV) vector injection.
• The imaging experimental protocol takes approximately 8 h from the beginning of brain slice preparation to completion of 2PLSM imaging.
• The described protocol, from slice preparation to signal analysis, can also be adapted for astrocyte Ca2+ experiments using epifluorescence or confocal microscopy.
Keywords: Astrocytes (星形胶质细胞)Graphical overview
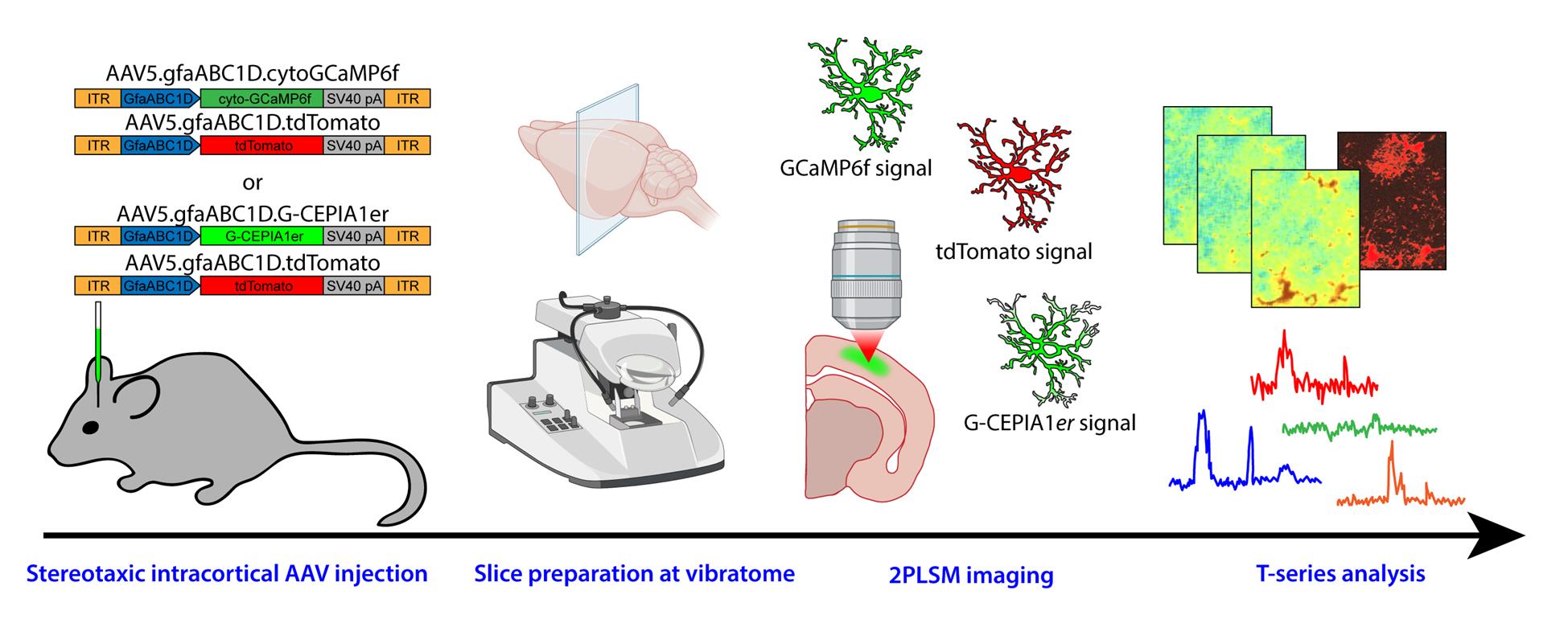
Graphical overview of the protocol from adeno-associated viral (AAV) vector injection to image analysis
Background
Astrocytes have been recognized by several studies as active partners of neurons in brain physiology [1–3]. Accordingly, their role has also been extensively investigated in relation to various pathological states of the central nervous system, to gather information on their possible contribution to pathogenesis and to explore the intriguing possibility that targeting astrocytes may support neuronal recovery [4]. Regardless of the specific research aim, a commonly used proxy for activation of astrocytes is the occurrence of transient increases in the cytosolic Ca2+ concentration. Neurotransmitters and neuromodulators such as glutamate, ATP, acetylcholine, and noradrenaline activate specific receptors on the plasma membrane of astrocytes, triggering a cascade of responses that often leads to an increase in the cytosolic Ca2+ concentration [5]. These Ca2+ elevations in astrocytes contribute to gliotransmitter release, thereby influencing the surrounding brain microenvironment and affecting neuronal transmission, brain circuits, and ultimately behavior [6].
This is the rationale for the development of methodological approaches designed to dynamically monitor Ca2+ signals in astrocytes from different tissue preparations and intracellular compartments. Ca2+ imaging is routinely performed on astrocytes from acute brain slices in many laboratories, either by loading cells with chemical Ca2+ probes (e.g., Fluo-4 AM) primarily labeling the cell somata [7–9] or by inducing targeted expression of genetically encoded indicators (e.g., GCaMP), which provide information on different subcellular domains, including fine astrocyte processes [10–11]. While some chemical probes (e.g., Oregon Green BAPTA-1) allow the extraction of absolute Ca2+ concentrations through fluorescence lifetime microscopy [12], the analysis of Ca2+ fluctuations in most studies is relative to the changes in probe fluorescence and does not provide absolute concentration values. Current imaging approaches range from epifluorescence microscopy, which collects the fluorescence from all focal planes in the tissue depth, with superficial cells contributing more to the signal, to confocal microscopy, which refines the signal by restricting fluorescence collection from a single focal plane (or from a limited number of planes) with a pinhole of variable aperture, and to 2-photon laser scanning microscopy (2PLSM), which ensures imaging confocality and optical sectioning through single-plane excitation [9,13,14]. Indeed, 2PLSM engages the concurrent illumination of a single focal plane with two low-energy photons, which combine their energy to allow excitation of the fluorophore only in that precise focal plane, thus considerably lowering tissue photodamage. Here, we report the procedures related to preparation and 2P imaging of coronal cortical slices from adeno-associated viral (AAV) vector-injected adult mice, which allow us to provide data on cytosolic Ca2+ fluctuations, with the GCaMP6f probe, and on ER Ca2+ resting state and dynamics, with the G-CEPIA1er probe.
Materials and reagents
Biological materials
1. Adeno-associated viral (AAC) vectors:
a. AAV5.GfaABC1D.tdTomato.SV40 (Penn Vector Core, Addgene 44332; MTA required by Addgene)
b. AAV5.GfaABC1D.cyto-GCaMP6f.SV40 (Penn Vector Core, Addgene 52925; MTA required by Addgene)
b. AAV5.GfaABC1D.G-CEPIA1er.SV40 [this plasmid is not commercially available; it was kindly donated by Dr. Y. Okubo (Department of Pharmacology, Graduate School of Medicine, University of Tokyo). Note that a virus packaging service is needed to insert the plasmid into the AAV5 vector].
Note: Store AAVs at -80 °C upon arrival. Prepare aliquots of appropriate volume (e.g., 3–5 μL) from the delivered batch to avoid freezing/thawing cycles.
Critical: Use a minimum titer of 1–2 × 1013 vg/mL.
Reagents
1. Phosphate buffer saline (PBS), sterile-filtered, pH 7.4 (Merck, catalog number: P4474)
2. Carprofen (Rimadyl, Pfizer, USA)
3. Eye ointment (Paralube ophthalmic ointment or similar, e.g., Lubrithal)
3. Povi-iodine 100 (Farmec srl) or similar (e.g., Betadine)
4. Isoflurane (Iso-Vet, Boehringer Ingelheim Animal Health Italia S.p.A)
5. Glucose (Merck, catalog number: G7528)
6. NaCl (Merck, catalog number: S7653)
7. KCl (Merck, catalog number: P9333)
8. NaHCO3 (Merck, catalog number: S5761)
9. NaH2PO4 (Merck, catalog number: S3139)
10. MgCl2 (Merck, catalog number: M2670)
11. CaCl2 (Merck, catalog number: 21115)
12. K-Gluconate (Merck, catalog number: P1847)
13. EGTA (Merck, catalog number: E4378)
14. HEPES (Merck, catalog number: H3375)
15. D-Mannitol (Merck, catalog number: M4125)
Note: Chemicals and salts may also be purchased from other companies.
Solutions
1. Standard solution (see Recipes)
2. Gluconate solution (see Recipes)
3. Mannitol solution (see Recipes)
4. Recording solution (see Recipes)
Recipes
1. Standard solution (final volume of 500 mL)
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Glucose | 25 mM | 2.253 g |
| NaCl 2 M | 125 mM | 31.25 mL |
| KCl 2 M | 2.5 mM | 0.625 mL |
| NaHCO3 0.5 M | 25 mM | 25 mL |
| NaH2PO4 1 M | 1.25 mM | 0.625 mL |
| MgCl2 1 M | 1 mM | 0.5 mL |
| CaCl2 1 M | 2 mM | 1 mL |
Note: Bring to a final volume of 500 mL with MilliQ water and keep the solution on ice. Before adding CaCl2, bubble the solution for at least 20 min with 95% O2 and 5% CO2 to reach pH 7.4 and prevent CaCl2 precipitation. Keep constantly bubbled throughout the preparation. Prepare fresh before use. A 10× stock solution (500 mL) can be prepared without glucose, MgCl2, and CaCl2. Store the 10× solution at 4 °C and use it within 1 month.
2. Gluconate solution (2 L stock)
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Glucose | 25 mM | 9.01 g |
| K-Gluconate | 130 mM | 60.9 g |
| KCl | 15 mM | 2.24 g |
| EGTA | 0.2 mM | 0.152 g |
| HEPES | 20 mM | 9.53 g |
Note: Adjust the pH to 7.4 with NaOH and bring to a final volume of 2 L. This solution can be prepared in advance as 1× stock and frozen at -20 °C in 50 mL tubes for up to 6 months. On the day of the experiment, you will need 100 mL of solution; thus, thaw two aliquots and keep the solution on ice under 99.95% O2 bubbling for at least 45 min before use. Keep the solution bubbled with 99.95% O2 throughout the procedure to ensure proper oxygenation of the tissue. Since this solution is buffered with HEPES and not with NaHCO3, it does not require CO2 bubbling.
3. Mannitol solution (final volume of 100 mL)
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Glucose | 25 mM | 0.45 g |
| D-Mannitol | 225 mM | 4.1 g |
| KCl 2 M | 2.5 mM | 0.125 mL |
| NaHCO3 0.5 M | 26 mM | 5.2 mL |
| NaH2PO4 1 M | 1.25 mM | 0.125 mL |
| MgCl2 1 M | 8 mM | 0.8 mL |
| CaCl2 1 M | 0.8 mM | 0.08 mL |
Note: Bring to a final volume of 100 mL with MilliQ water. Before adding CaCl2, bubble the solution for at least 20 min with 95% O2 (ensure proper oxygenation of the tissue) and 5% CO2 to reach pH 7.4, thus preventing CaCl2 precipitation. Keep constantly bubbled at room temperature throughout the preparation. Prepare fresh before use. A 10× stock solution (500 mL) can be prepared without glucose, mannitol, MgCl2, and CaCl2. Store the 10× solution at 4 °C and use it within 1 month.
4. Recording solution (500 mL)
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Glucose | 10 mM | 0.9 g |
| NaCl 2 M | 120 mM | 30 mL |
| KCl 2 M | 2.5 mM | 0.625 mL |
| NaHCO3 0.5 M | 26 mM | 26 mL |
| NaH2PO4 1 M | 1.25 mM | 0.625 mL |
| MgCl2 1 M | 1 mM | 0.5 mL |
| CaCl2 1 M | 1 mM | 0.5 mL |
Note: Bring to a final volume of 500 mL with MilliQ water. Before adding CaCl2, bubble the solution for at least 20 min with 95% O2 and 5% CO2 to reach pH 7.4 and prevent CaCl2 precipitation. Keep constantly bubbled throughout the preparation. Prepare fresh before use. Keep the solution at room temperature.
Laboratory supplies
1. Surgery tools: scissors, forceps, tweezers, suture thread [Fine Science Tools: catalog number: 14060-09 (fine scissors, sharp), 11210-10 (Dumont AA, epoxy-coated forceps), 15018-10 (Vannas spring scissors, Ethicon), 8697 6-0 (Prolene suture thread)]
2. Glass capillaries (pipettes) (Narishige, catalog number: GD-1)
3. Drill burs (Meisinger, catalog number: 1RF-007; Fine Science Tools, catalog number: 19008-07 SS 0.7 mm, round HP Burs)
4. Cotton swabs
5. Beakers (50 mL and 100 mL)
6. Bottles (100 mL and 500 mL) for saline solutions
Equipment
1. Freezer -80 °C (Thermo Scientific, model: Revco ExF)
2. Freezer -20 °C (Liebherr, model: SFFvh 4001 Perfection)
3. Refrigerator 4 °C (Liebher, model: SRFfg 3501 Performance)
4. Thermostatic bath (ISCO, model: GTR-90)
5. Pure O2 tank (AirLiquide, Oxygen purity ≥ 99,995%)
6. 5% CO2/95% O2 tank (AirLiquide)
7. Pipette puller (Narishige, Japan, model: PC-10)
8. Anesthesia unit for isoflurane (anesthesia system, lab animal, complete, V-1 tabletop with active scavenging) (KF Technology, model: 901820)
9. Heating pad with controller (Stoelting, model: 53800M)
10. Digital stereotaxic apparatus (digital just for mouse stereotaxic instrument) (Stoelting, model: 51730D)
11. Stereomicroscope (Leica, model: CLS150MR)
12. Dental drill (Sifradent, model: MM 330VPR)
13. Heat lamp (Braintree Scientific Inc., model: MM 330VPR); as a general recommendation, use an infrared heat lamp with a 150–250 W bulb and an adjustable swing arm for easy adjustment of the distance to the animal. The animal should have the possibility to move away to a non-heated part of the cage
14. Vibratome (Leica BioSystems, model: VT1200)
15. Dual Fluorescent Protein Flashlight kit, including barrier filter glasses (NightSea, now replaced by Xite Flashlight System)
16. 2-photon laser scanning microscope (Multiphoton Imaging System, Scientifica Ltd., UK) equipped with a water-immersion objective (LUMPlan FI/IR 20×, 1.05 NA, Olympus), a pulsed mode locked Ti:Sapphire laser (Chameleon Ultra 2, Coherent, USA) and a Pockels cell for laser power modulation (ConOptics, USA, model: 302RM)
17. Peristaltic pump (Ismatec ISM831C Reglo Digital Variable-Speed Peristaltic Pump 2 Channel)
Software and datasets
1. SciScan (Scientifica UK, version 1.2, included with Scientifica 2-photon system) for image acquisition
2. ImageJ (publicly available at https://github.com/imagej/ImageJ, we used version 1.51n) for image analysis
3. GECI Quant script (see https://baljitkhakhlab.healthsciences.ucla.edu/tools) for ROI detection in ImageJ
4. AstroResp Code (publicly available at https://github.com/ladymariot/AstroResp) for peak detection, extraction of fluorescent traces, and peak parameters
5. MATLAB 7.6.0 R2008 A (Mathworks, Natick, MA, USA) for running AstroResp Code
6. Excel (Microsoft Office Professional 2016, OpenOffice Calc as a free alternative) for data handling
7. OriginPro (OriginLab, version 2018, QtiPlot as a free alternative) for trace analysis and graph production
Procedure
文章信息
稿件历史记录
提交日期: Mar 31, 2025
接收日期: May 22, 2025
在线发布日期: Jun 18, 2025
出版日期: Jul 5, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Lia, A. and Zonta, M. (2025). Two-photon (2P) Microscopy to Study Ca2+ Signaling in Astrocytes From Acute Brain Slices. Bio-protocol 15(13): e5371. DOI: 10.21769/BioProtoc.5371.
分类
神经科学 > 细胞机理 > 胞内信号传导
细胞生物学 > 细胞成像 > 双光子显微镜
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link