- EN - English
- CN - 中文
Evaluation of In Vitro Cytotoxic Activity of CAR-T Cells Using Patient-Derived Organoids
基于患者类器官的CAR-T细胞体外毒性活性评价
发布: 2025年07月05日第15卷第13期 DOI: 10.21769/BioProtoc.5369 浏览次数: 2394
评审: Dipak Kumar PoriaMartin V KolevSamuele Tardito

相关实验方案
髓系肿瘤中用于生殖系 DNA 分离的皮肤成纤维细胞标准化培养:一种从床旁到实验室的实用方法
Parampreet Kour [...] Pulkit Rastogi
2025年10月05日 1239 阅读
Abstract
Adoptive immune cell therapy, especially chimeric antigen receptor T (CAR-T) cells, has emerged as a promising strategy in solid tumor treatment, owing to its unique ability to specifically recognize and effectively eliminate tumor cells. Patient-derived organoids (PDOs) offer a robust and physiologically relevant platform for assessing the safety and efficacy of CAR-T-cell-based therapies. We now describe a detailed protocol for an in vitro evaluation system based on the co-culture of PDOs and CAR-T cells. This system encompasses the establishment of tumor organoids from patient tumor specimens, the isolation of T cells from matched peripheral blood mononuclear cells (PBMCs), and the generation of antigen-specific CAR-T cells. Through the use of fluorescent labeling to visualize different cells and apoptosis-related events post-interaction, along with quantitative analyses of T-cell proliferation, tumor organoid apoptosis, and the secretion of immune effector molecules, this system enables a robust and multifaceted evaluation of CAR-T cell cytotoxicity in vitro. Collectively, this co-culture system provides a systematic and reproducible in vitro platform for evaluating the functional activity of CAR-T cells and advancing research in tumor immunology and immunotherapy.
Keywords: Patient-derived organoids (患者来源类器官)Graphical overview
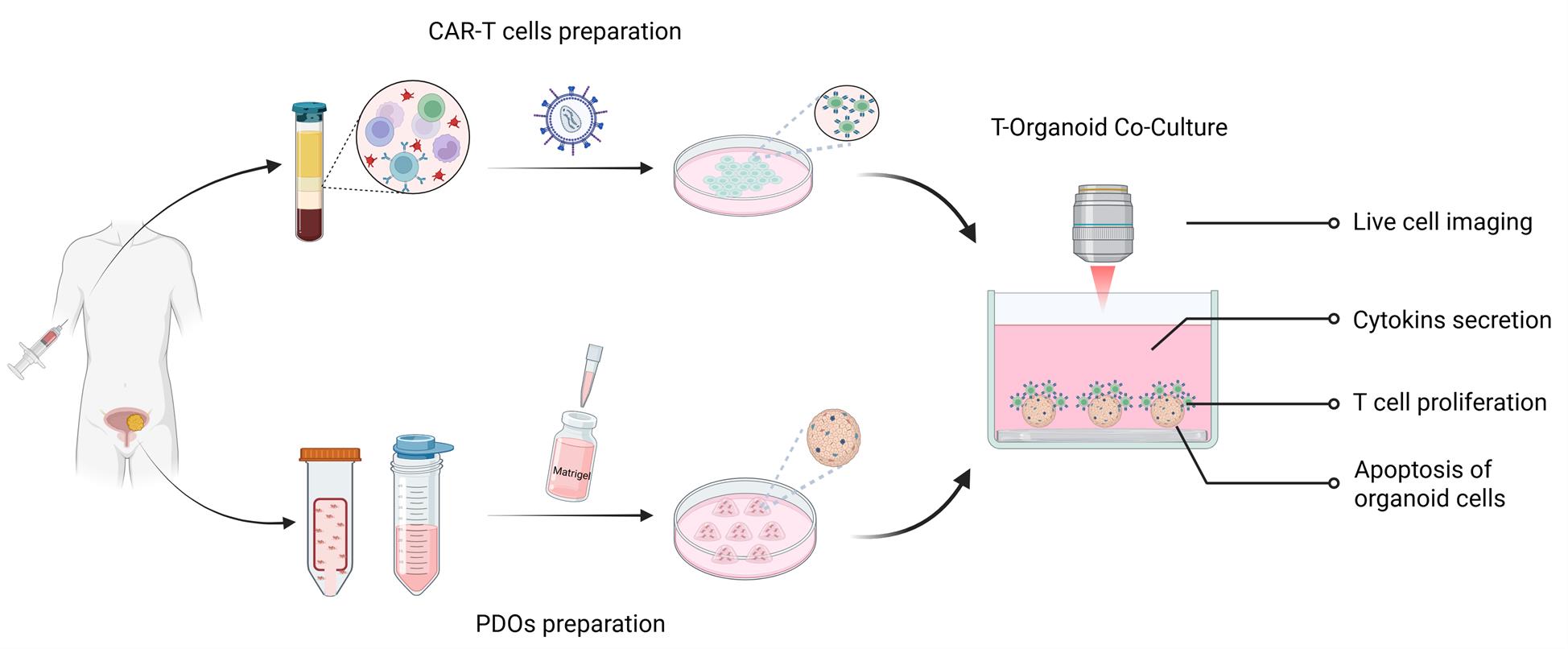
Background
Patient-derived organoids (PDOs) not only preserve the heterogeneity of the original tumor cells but also retain a diverse range of oncological characteristics. As a result, they have become a powerful model for accurately predicting clinical drug sensitivity and providing a reliable platform for preclinical drug development and validation [1]. However, existing tumor organoid culture systems have inherent limitations. The absence of an immune system poses significant challenges in evaluating immune-based therapies. To address this, immune cell–tumor organoid co-culture models have emerged as a valuable complementary approach. These models offer a high-fidelity platform for investigating cell–cell interactions within the tumor microenvironment and advancing the development of novel cancer immunotherapies [2].
CAR-T cell therapy leverages genetic engineering to modify T cells, enabling them to specifically recognize tumor antigens and precisely target cancer cells. This approach offers new hope to patients with solid tumors that are unresponsive to conventional treatments [3]. The development of every effective CAR-T cell therapy requires extensive preclinical evaluation and validation. Organoids preserve key characteristics such as tumor cell heterogeneity, making them a more accurate model of in vivo tumor conditions compared to traditional tumor cell lines [4].
In our previous investigations, we demonstrated that PDOs reliably recapitulate the antigen expression profiles of their corresponding primary tumor tissues. Owing to this, PDOs represent a valuable platform for the preclinical in vitro evaluation of chimeric antigen receptor T (CAR-T) cell therapies [5,6]. In the present study, we summarize and refine a streamlined in vitro co-culture system for assessing CAR-T cell function. This work aims to advance the broader application of organoid models in diverse tumor immunology and immunotherapy research contexts.
Materials and reagents
Reagents
1. DPBS, no calcium, no magnesium (Gibco, catalog number: 14190144)
2. AlbumiNZTM bovine albumin low IgG (BSA) (MP Biomedicals, catalog number: 02199897-CF)
3. Advanced DMEM/F-12 (Gibco, catalog number: 12634010)
4. Antibiotic-Antimycotic (Gibco, catalog number: 15240062)
5. GlutaMAX supplement (Gibco, catalog number: 35050061)
6. HEPES (1 M) (Thermo Fisher, catalog number: 15630080)
7. B-27 Supplement (50×), serum free (Gibco, catalog number: 17504044)
8. Fetal bovine serum (FBS) (Gibco, catalog number: 10091148)
9. Recombinant human R-spondin 1 protein (R&D Systems, catalog number: 4645-RS-250)
10. Recombinant human Noggin protein (Peprotech, catalog number: 120-10C-1000)
11. Recombinant human EGF protein (Peprotech, catalog number: 100-15)
12. Recombinant human basic FGF protein (Peprotech, catalog number: 100-18B)
13. Recombinant human FGF-10 protein (Peprotech, catalog number: 100-26)
14. Y-27632 (Abmole, catalog number: M1817)
15. N-Acetylcysteine (Sigma, catalog number: A9165)
16. Nicotinamide (Sigma, catalog number: N0636)
17. SB202190 monohydrochloride hydrate (Sigma, catalog number: S7076)
18. A83-01 (Sigma, catalog number: SML0788)
19. Matrigel® growth factor reduced (GFR) basement membrane matrix, LDEV free (Corning, catalog number: 356231)
20. Collagenase, type II, powder (Gibco, catalog number: 17101015)
21. DNase I (Roche, catalog number: 11284932001)
22. TrypLE Express (Gibco, catalog number: 12605036)
23. CD3 microbeads (Miltenyi Biotec, catalog number: 130-097-043)
24. OctoMACSTM separator (Miltenyi Biotec, catalog number: 130-042-109)
25. Dynabeads human T-activator CD3/CD28 for T-cell expansion and activation (Gibco, catalog number: 11131D)
26. Recombinant human IL-2 protein (Peprotech, catalog number: 200-02)
27. Recombinant human IL-15 protein (Peprotech, catalog number: 200-15)
28. X-VIVO 15 serum-free medium (LONZA, catalog number: 04-418Q)
29. Propidium iodide (Solarbio, catalog number: C0080)
30. Hoechst 33342 (Solarbio, catalog number: C0031)
31. CFSE (Invitrogen, catalog number: 65085084)
32. 0.5 M EDTA (pH 8.0) (Solarbio, catalog number: E1170)
33. Penicillin–streptomycin (Gibco, catalog number: 15070063)
34. Ficoll-PaqueTM PLUS density gradient media (Cytiva, catalog number: 17144002)
35. Authentikine TNF-alpha ELISA kit (Proteintech, catalog number: KE00154)
36. OptEIA Human IFN-γ ELISA kit (BD Pharmingen, catalog number: 550612)
37. Cleaved Caspase-3 (Asp175) ELISA kit (Abcam, catalog number: ab220655)
38. Anti-MUC1 antibody (Cell Signaling Technology, catalog number: 4358S)
39. Goat anti-mouse IgG (H+L) highly cross-absorbed secondary antibody, Alexa Fluor 555 (Thermo Fisher, catalog number: A21422)
40. G4S linker (E702V) rabbit mAb, Alexa Fluor 488 conjugate (Cell Signaling Technology, catalog number: 50515)
41. Recovery cell culture freezing medium (Gibco, catalog number: 12648010)
42. Lentiviral vector encoding a CAR sequence composed of MUC1 scFV (HMFG2), an IgD hinge, CD28 transmembrane domain, 4-1BB, and CD3ξ signaling domains, synthesized by Hanbio, China. The corresponding lentivirus, with a titer exceeding 1 × 108 TU/mL, was also produced by the vendor. Viral aliquots were stored at -80 until use.
43. NH4Cl (Aladdin, catalog number: A116373)
44. KHCO3 (Aladdin, catalog number: P639954)
45. Antifade solution (Sigma, catalog number: S7114)
46. Neutral buffered formalin (Solarbio, catalog number: G2161)
47. TBST buffer (Solarbio, catalog number: T1085)
Solutions
1. Organoid culture basal medium (see Recipes)
2. Bladder cancer organoid medium (see Recipes)
3. CAR-T cell culture medium (see Recipes)
4. MACS buffer (see Recipes)
5. Organoid recovery solution (see Recipes)
6. Tissue digestion solution (see Recipes)
7. RBC lysis buffer (see Recipes)
Recipes
1. Organoid culture basal medium
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Advanced DMEM/F-12 | n/a | top up to 500 mL |
| HEPES | 1% | 5 mL |
| GlutaMAX | 1% | 5 mL |
| Antibiotic-Antimycotic | 1% | 5 mL |
| Total | n/a | 500 mL |
2. Bladder cancer organoid medium
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Organoid culture basal medium | n/a | top up to 100 mL |
| B-27 supplement | 1× | 5 mL |
| N-acetylcysteine | 1.25 mM | 250 μL |
| Nicotinamide | 10 mM | 1 mL |
| SB202190 | 10 μM | 33.4 μL |
| A83-01 | 500 nM | 5 μL |
| Recombinant human R-spondin 1 | 500 ng/mL | 500 μL |
| Recombinant human noggin | 100 ng/mL | 100 μL |
| Recombinant human FGF-10 | 20 ng/mL | 20 μL |
| Recombinant human FGF-2 | 5 ng/mL | 5 μL |
| Recombinant human EGF | 50 ng/mL | 50 μL |
| Y-27632* | 10 μM | 5 μL |
| Total | n/a | 100 mL |
*Only required for initial derivation of organoids (first passage)
3. CAR-T cell culture medium
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| RPMI 1640 | n/a | top up to 100 mL |
| FBS | 10% | 10 mL |
| Penicillin-streptomycin | 1% | 5 mL |
| Recombinant human IL-2 | 50 IU/mL | 5 μL |
| Recombinant human IL-15 | 1 ng/mL | 1 μL |
| Total | n/a | 100 mL |
4. MACS buffer
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| DPBS | n/a | top up to 100 mL |
| BSA | 0.5% | 0.5 g |
| EDTA (pH 8.0) | 2 mM | 400 μL |
| Total | n/a | 100 mL |
5. Organoid recovery solution
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| DPBS | n/a | top up to 100 mL |
| EDTA (pH 8.0) | 10 mM | 2 mL |
| Y27632 | 10 μM | 10 μL |
| Total | n/a | 10 mL |
6. Tissue digestion solution
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Advanced DMEM/F-12 | n/a | top up to 50 mL |
| Collagenase II | 5 mg/mL | 1 mL |
| DNAse I | 100 μg/mL | 0.5 mL |
| Y27632 | 10 μM | 5 μL |
| Total | n/a | 50 mL |
7. RBC lysis buffer
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| NH4Cl | 150 mM | 1.208 g |
| KHCO3 | 10 mM | 0.1 g |
| EDTA (500 mM pH 8.0) | 100 μM | 20 μL |
| Total | n/a | 100 mL |
Laboratory supplies
1. Costar 6-well clear TC-treated multiple well plates (Corning, catalog number: 3516)
2. Costar 24-well clear TC-treated multiple well plates (Corning, catalog number: 3738)
3. Costar 48-well clear TC-treated multiple well plates (Corning, catalog number: 3548)
4. 10/200/1,000 μL universal pipette tips (Axygen, catalog number: T-300, T-200-C-R-S, T-1000-B-R-S)
5. 15 mL PP centrifuge tubes (Corning, catalog number: 340052)
6. 50 mL PP centrifuge tubes (Corning, catalog number: 430290)
7. 70 μm cell strainer (Corning, catalog number: 352350)
8. 100 mm TC-treated culture dish (Corning, catalog number: 430167)
9. Falcon® round-bottom polystyrene tubes (Corning, catalog number: 352235)
10. Cryogenic vials (Corning, catalog number: A38053)
Equipment
1. Biological safety cabinet (Heal Force, catalog number: HFsafe-1200LC)
2. Centrifuge (Eppendorf, catalog number: 5702)3. Inverted microscope (Olympus, catalog number: CKX53)
4. Constant temperature water bath (Shanghai Yiheng, catalog number: HWS-24)
5. Carbon dioxide incubator (Thermo Fisher, catalog number: 3110)
6. Ice maker (Xuehua, catalog number: IMS-40)
7. Shaking incubator (Shanghai Shiping, catalog number: SPH-211B)
8. Cell counter (Thermo Fisher, catalog number: Countess IIFL)
9. Multifunctional microplate reader (PerkinElmer, catalog number: VICTOR Nivo)
10. Confocal microscope (Nikon, catalog number: AX)11. Flow cytometer (BD Biosciences, catalog number: FACS ARIA )
Procedure
文章信息
稿件历史记录
提交日期: Apr 7, 2025
接收日期: Jun 3, 2025
在线发布日期: Jul 2, 2025
出版日期: Jul 5, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Yu, L. (2025). Evaluation of In Vitro Cytotoxic Activity of CAR-T Cells Using Patient-Derived Organoids. Bio-protocol 15(13): e5369. DOI: 10.21769/BioProtoc.5369.
分类
免疫学 > 免疫疗法 > CAR-T
癌症生物学 > 肿瘤免疫学 > 癌症治疗
细胞生物学 > 细胞分离和培养 > 共培养
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
提问指南
+ 问题描述
写下详细的问题描述,包括所有有助于他人回答您问题的信息(例如实验过程、条件和相关图像等)。
Share
Bluesky
X
Copy link