- EN - English
- CN - 中文
Live Cell Imaging to Monitor Axonal Pruning in Drosophila Motor Neurons
果蝇运动神经元轴突修剪的活细胞成像观察方法
(*contributed equally to this work) 发布: 2025年07月05日第15卷第13期 DOI: 10.21769/BioProtoc.5367 浏览次数: 2027
评审: Oneil Girish BhalalaRupkatha BanerjeeAmr Galal Abdelraheem IbrahimAnonymous reviewer(s)
Abstract
Over the lifespan of an individual, brain function requires adjustments in response to environmental changes and learning experiences. During early development, neurons overproduce neurite branches, and neuronal pruning removes the unnecessary neurite branches to make a more accurate neural circuit. Drosophila motoneurons prune their intermediate axon bundles rather than the terminal neuromuscular junction (NMJ) by degeneration, which provides a unique advantage for studying axon pruning. The pruning process of motor axon bundles can be directly analyzed by real-time imaging, and this protocol provides a straightforward method for monitoring the developmental process of Drosophila motor neurons using live cell imaging.
Key features
• Long-range projecting axon bundles of Drosophila motor neurons extending from soma on the ventral nerve cord (VNC) undergo degeneration rather than retraction during metamorphosis.
• The pruning process of motor axon bundles can be directly observed by real-time live-cell imaging.
• The complete clearance of axon bundles occurs approximately 22 h after pupal formation (22 h APF).
• Mushroom body (MB) γ neuron axon pruning regulatory genes are conserved for motor neurons.
Keywords: Neurodevelopment (神经发育)Graphical overview
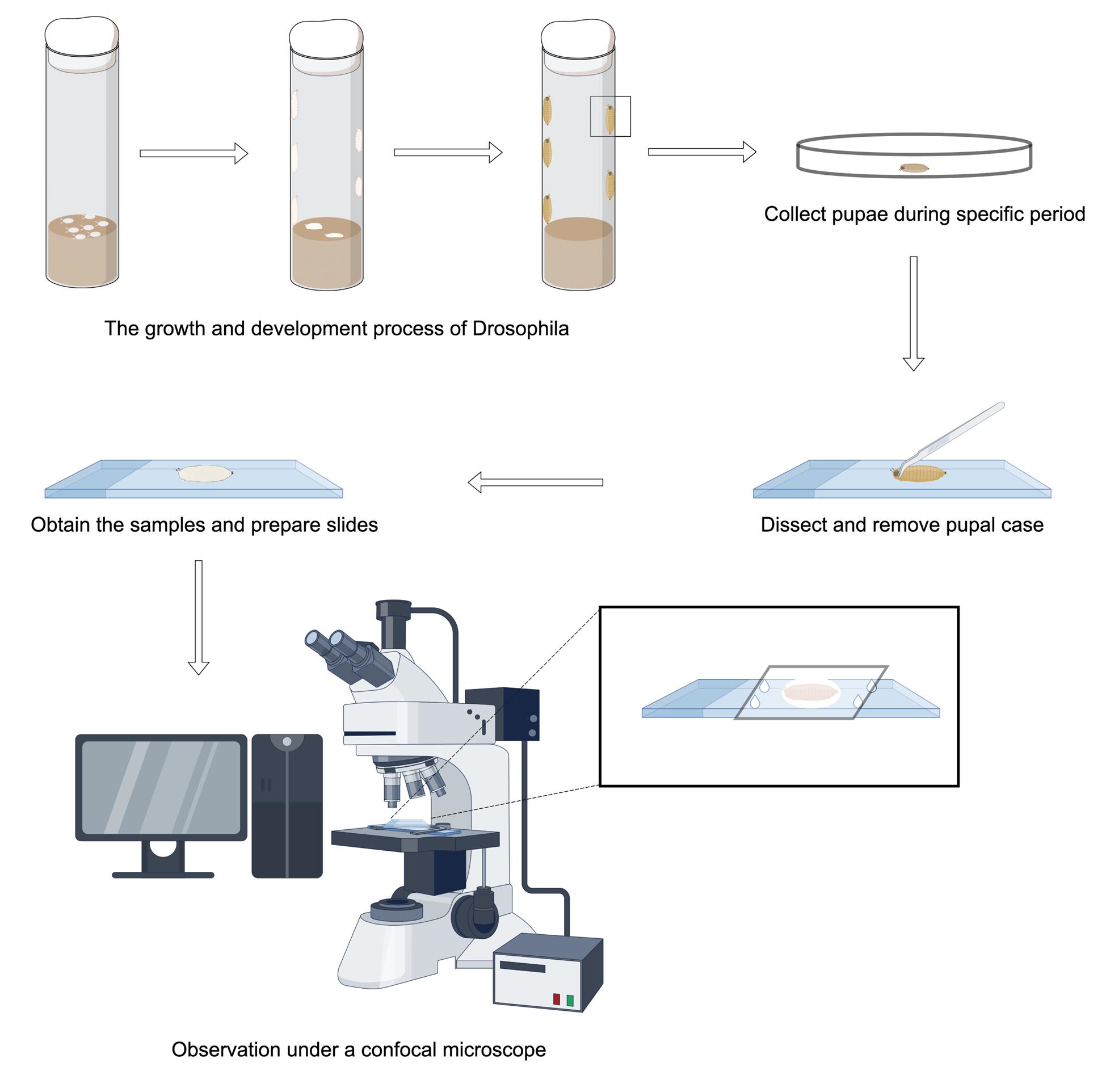
Background
During the early stages of nervous system development, nerve branches and connections are excessively generated. This includes a number of branches of axons and dendrites far greater than what is needed in an adult individual. Neural pruning is the process by which unnecessary synaptic connections between neural cells are selectively eliminated during the development of the nervous system [1,2]. This process occurs primarily in childhood and adolescence and is an essential mechanism for the brain to efficiently and optimally function as it matures. It is worth noting that numerous neurodevelopmental disorders, like autism spectrum disorder (ASD) and schizophrenia, are considered to be correlated with aberrant neural pruning [3–7]. In autistic individuals, it was discovered that insufficient pruning of neuronal connections in certain areas of the brain may lead to abnormal functioning of neural circuits. Conversely, excessive pruning is a possible cause of schizophrenia [8]. By investigating the mechanisms of abnormal neural pruning, we can deeply explore the roots of the pathogenesis of these disorders.
During metamorphosis in the holometabolous insect Drosophila, early neurons undergo stereotypic pruning to re-establish adult-specific neural circuits. One widely recognized type IV dendritic arborization (C4da) sensory neuron that perceives pain in Drosophila undergoes dendrite-specific pruning during development [9–12]. In addition, the dendrites and axons of the mushroom body (MB) γ neuron in the central nervous system (CNS) of Drosophila are also known to undergo pruning [13–15]. Although MB γ neurons provide fundamental insights into the mechanisms of axonal pruning, they require fixed tissue analysis and present imaging challenges in dense central brains. Here, we describe a novel method to monitor the process of axonal pruning in Drosophila motor neurons. Using live-cell imaging, we found that axons in the middle part of Drosophila motoneurons complete their pruning by degradation during development, while their terminal neuromuscular junctions are pruned by retraction [16]. Thus, motor-neuron axon bundles project peripherally in a stereotyped array, allowing for real-time live imaging, providing characteristic temporal windows and mechanistic properties (fragmentation or degeneration), and supporting systematic genetic screening to find new regulators. On the other hand, the development of Drosophila motor neurons as a model for axon pruning complements the MB γ neuron system by offering a distinct neuronal context, technical advantages, and functional readouts. Overall, the establishment of this method provides an ideal platform for future investigations into the regulatory mechanisms of axon pruning.
Materials and reagents
Reagents
1. 20× PBS (Sangon Biotech, catalog number: B548117-0500)
Note: Prior to observation, pupae are washed repeatedly 3–4 times with PBS [500 mL of 20× PBS stock solution is diluted to 10 L with H2O to become 1× PBS; storage conditions: 25 °C (room temperature)] buffer to remove surface debris such as adherent food.
2. Glycerol, 90% (Sangon Biotech, catalog number: 56-81-5); store at room temperature
3. Silicone resin (DOW CORNING-High vacuum grease); store at room temperature
Note: An appropriate amount of silicone resin is squeezed around the perimeter of the slide with the sample in the center to maintain a flexible gap between the slide and the coverslip to prevent the sample from being pressed and damaged.
4. OK6-Gal4 (Bloomington Stock Center, catalog number: BSC64199)
5. EcR-RNAi (Bloomington Stock Center, catalog number: BSC9327)
6. UAS-EcR-B1DN (Bloomington Stock Center, catalog number: BSC9452)
5. UAS-Uba1-RNAi (Tsinghua RNAi Stock Center, catalog number: THU2127)
6. UAS-mCD8-GFP (Su Wang; Southeast University, China)
Laboratory supplies
1. 200 and 1,250 μL pipette tips (Biosharp, catalog number: BS-RT)
2. 10 μL pipette tips (A-gen, catalog number: T-10-RS)
3. Glass culture dish (Normax, catalog number: 5058541)
4. 1.5 mL microcentrifuge tubes (Sangon Biotech, catalog number: F601620-0010)
5. Microscope slides (CITOTEST, catalog number: 1A5105)
6. Microscope cover glass (CITOTEST, catalog number: 10212432c)
Equipment
1. Pipettes (Eppendorf, model: Research® plus, catalog number: 3120000062) are used to clean the surface debris of Drosophila pupae
2. Stereomicroscope (Motic, model: SMZ168) is used to observe, dissect, and isolate samples from the period after pupal formation (APF)
3. Confocal laser scanning microscope (Carl Zeiss, model: LSM900) is used to image axon bundles in motor neurons
4. Anatomical forceps (Dumont, catalog number: 0209-5-PO) are used to dissect and isolate samples from the APF period
5. Anatomical scissors (RWD, catalog number: S11037-08) are used to assist in dissecting samples from the APF period
Software and datasets
1. Image J (Fiji; https://imagej.net/software/fiji/)
2. Prism 9 (GraphPad Prism 9.0)
3. ZEN (2012 blue edition)
4. Adobe Photoshop (2018)
5. Adobe Illustrator (2024)
Procedure
文章信息
稿件历史记录
提交日期: Mar 2, 2025
接收日期: Jun 3, 2025
在线发布日期: Jun 19, 2025
出版日期: Jul 5, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Long, K., Xu, W., Miao, X., Wang, S. and Rui, M. (2025). Live Cell Imaging to Monitor Axonal Pruning in Drosophila Motor Neurons. Bio-protocol 15(13): e5367. DOI: 10.21769/BioProtoc.5367.
分类
神经科学 > 发育 > 神经元
神经科学 > 细胞机理 > 神经元命运
细胞生物学 > 细胞成像 > 活细胞成像
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link