- EN - English
- CN - 中文
Protocol for Generation of Single-Gene Knockout in Hard-to-Transfect THP1 Cell Lines Using CRISPR/Cas9
利用CRISPR/Cas9在难转染的THP1细胞系中构建单基因敲除的实验方案
发布: 2025年07月05日第15卷第13期 DOI: 10.21769/BioProtoc.5365 浏览次数: 3507
评审: Pooja MukherjeeAnonymous reviewer(s)
Abstract
This protocol provides a step-by-step approach for generating single-gene knockout in hard-to-transfect suspension immune cell lines like THP1, specifically demonstrated by knocking out the GSDMD gene. By employing CRISPR-Cas9 system delivered via lentivirus, this protocol enables precise gene disruption through targeted single-guide RNAs (sgRNAs). Key steps include designing specific sgRNAs, cloning them into a CRISPR vector, viral packaging, and transducing the target cells, followed by selection and validation. This optimized protocol is particularly useful for functional studies in immune cells, allowing researchers to reliably explore gene function in complex cellular pathways.
Key features
• CRISPR-Cas9-based knockout strategy tailored for hard-to-transfect THP1 cells using lentiviral delivery.
• Lentiviral transduction ensures stable gene delivery with high efficiency compared to other traditional methods like transfection and electroporation.
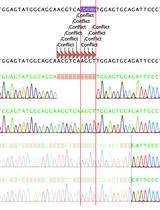
• Stepwise validation through colony PCR, sequencing, and western blotting to confirm successful knockout.
• Scalable approach, applicable to various cell models for functional genomic studies.
Keywords: CRISPR/Cas9 (CRISPR/Cas9)Graphical overview
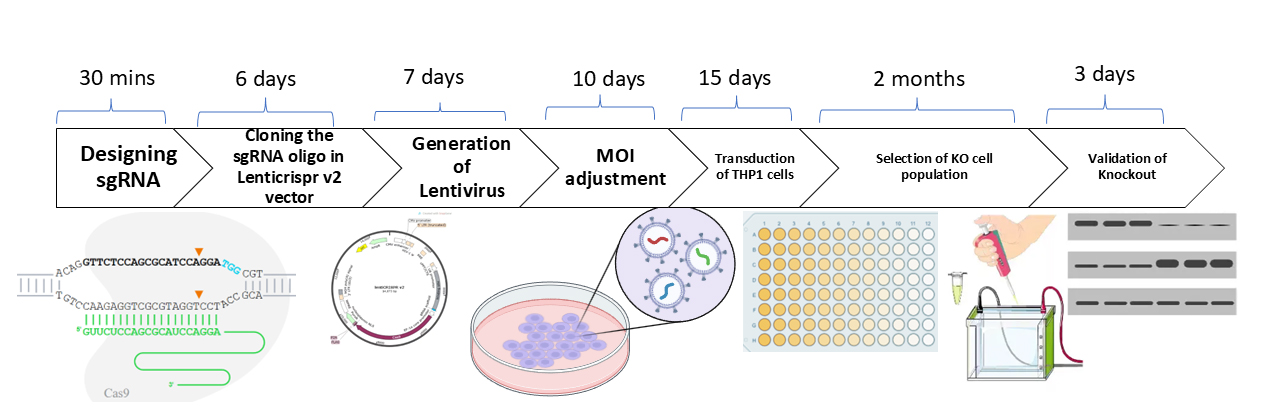
Background
The advent of CRISPR-Cas9 genome editing has revolutionized genetic research by providing a versatile and precise tool for targeted genetic modifications. CRISPR (clustered regularly interspaced short palindromic repeats) and its associated protein Cas9 function together as a programmable nuclease system. The Cas9 enzyme is guided by a single-guide RNA (sgRNA) to introduce double-strand breaks (DSBs) at specific genomic loci. These breaks are repaired by the cell through two major endogenous pathways: non-homologous end joining (NHEJ), an error-prone process that can introduce insertions or deletions (indels), and homology-directed repair (HDR), which can incorporate a donor DNA template for precise edits. However, the effective delivery of CRISPR components in hard-to-transfect suspension cells remains a challenge. Conventional transfection techniques, such as electroporation and chemical transfection, are inefficient with high levels of cytotoxicity, rendering them unsuitable for functional genomic applications [1,2].
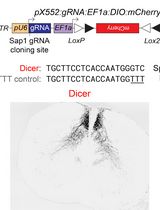
To overcome these limitations, lentiviral transduction offers a robust alternative, ensuring stable and efficient gene delivery into immune cell models. This protocol optimizes lentivirus-based CRISPR-Cas9 knockout strategies using sgRNA to achieve high knockout efficiency in THP1 cells, particularly targeting GSDMD, a key regulator in pyroptotic cell death pathways. This protocol builds upon and optimizes previously published protocols for creating single-gene knockouts in THP1 cells. This protocol provides improvements over earlier methods, namely the incorporation of LentiX cells, which are subcloned for high viral titer, and the involvement of GoStix-based viral titration, which offers a rapid and user-friendly alternative to more complex techniques like qPCR or ELISA [3]. In addition, the protocol streamlines puromycin selection and validation steps through a combination of colony PCR, Sanger sequencing, and western blotting. The approach is highly adaptable, offering a scalable method for gene knockout studies in immune cells and other hard-to-transfect models.
Materials and reagents
Biological materials
1. THP1 cells (ATCC, catalog number: TIB-202)
2. Lenti-X cells (Takara, catalog number: 632180)
3. LentiCRISPRv2 (Addgene, catalog number: 52961)
4. E. coli Stbl3 competent cells
5. PsPAX2 (Addgene, catalog number: 12260)
6. pMD2.G (Addgene, catalog number: 12259)
Reagents
1. T4 PNK (New England Biolabs, catalog number: M0201S)
2. T4 DNA ligase (New England Biolabs, catalog number: M0202)
3. Puromycin (Invitrogen, catalog number: A1113803)
4. Ampicillin (HiMedia, catalog number: MB104)
5. 10× PCR buffer (Sigma, catalog number: D4545)
6. Taq polymerase (Sigma, catalog number: D4545)
7. MgCl2 (Sigma, catalog number: D4545)
8. dNTPs (Thermo Fisher Scientific, catalog number: R0191)
9. T4 ligation buffer (New England Biolabs, catalog number: B0202S)
10. Polybrene (Sigma, catalog number: TR-1003-G)
11. Lipofectamine 2000 (Thermo Fisher Scientific, catalog number: 11668500)
12. PLUS reagent (Thermo Fisher Scientific, catalog number: 11514015)
13. Penstrep (Gibco, catalog number: 15140122)
14. Agarose (Invitrogen, catalog number: 16500500)
15. LentiX concentrator (Takara, catalog number: 631231)
16. Lenti Go stix (Takara, catalog number: 631280)
17. Methanol (SRL, catalog number: 65524)
18. 30% acrylamide:bisacrylamide (Bio-Rad, catalog number: 1610156)
19. Bovine serum albumin (BSA) (MP Biomedicals, catalog number: 0215240150)
20. APS (Bio-Rad, catalog number: 1610700)
21. BsmbI-v2 (New England Biolabs, catalog number: R0739S)
22. r3.1 buffer (New England Biolabs, catalog number: B6003S)
23. TEMED (Sigma-Aldrich, catalog number: 1107320100)
24. cOmplete Mini (Sigma-Aldrich, catalog number: 4693159001)
25. Polyvinylidene fluoride (PVDF) membrane (Merck, catalog number: IPVH00010)
26. Luminescent Cell Viability Assay kit (Promega, catalog number: G7571)
27. Clarity western ECL substrate (Bio-Rad, catalog number: 1705061)
28. LB broth (HiMedia, catalog number: M1245)
29. Opti-MEM reduced serum media (Gibco, catalog number: 31985062)
30. RPMI 1640 (HiMedia, catalog number: AL162A)
31. Trypsin-EDTA (0.25%) (Gibco, catalog number: 15400054)
32. DMEM (HiMedia, catalog number: AL0662A)
33. 1× PBS (HiMedia, catalog number: TL1006)
34. FBS (Gibco, catalog number: 16140071)
Solutions
1. 10× TBE buffer (see Recipes)
Recipes
1. 10× TBE buffer (pH 8.3)
| Component | Final concentration in 10× | Quantity |
|---|---|---|
| Tris base | 0.89 M | 108 g |
| Boric acid | 0.89 M | 55 g |
| 0.5 M EDTA (pH 8.0) | 20 mM | 40 mL |
| Distilled water | Up to 1 L |
Laboratory supplies
1. 96-well plates (Corning, catalog number: CLS3360)
2. 12-well plates (Corning, catalog number: CLS3513)
3. 6-well plates (Corning, catalog number: CLS3516)
4. 15 mL centrifuge tubes (Corning, catalog number: CLS430791)
5. 50 mL centrifuge tubes (Corning, catalog number: CLS430829)
6. 10 mL steripipettes (Corning, catalog number: CLS4488)
7. 90 mm Petri dishes (Corning, catalog number: CLS430166)
8. T75 flasks (Corning, catalog number: 430641U)
9. T25 flasks (Corning, catalog number: CLS431463)
10. 0.45 μm vacuum filter (Corning, catalog number: 430314)
Equipment
1. Thermal cycler (VeritiTM Thermal Cycler, 96-well Fast, catalog number: 4375305)
2. CO2 incubator (Thermo Fisher Scientific, FormaTM Steri-CycleTM CO2 Incubator, model: 371)
3. Centrifuge (Thermo Fisher Scientific, Sorvall legend X1R, catalog number: 75004241)
4. Dry bath incubator (Benchmark, catalog number: BSH1004)
5. Electrophoresis power supply (Bio-Rad, Mini-Sub Cell GT System, catalog number: 1704486EDU)
6. Gel documentation system (Bio-Rad, model: Gel Doc EZ Gel Documentation System)
7. Biosafety cabinet (Thermo Fisher Scientific, 1300 Series A2 Class II, model: 1386)
Software and datasets
1. Synthego CRISPR Design Tool (https://www.synthego.com)
2. BioEdit (for Sanger sequencing result visualization)
Procedure
文章信息
稿件历史记录
提交日期: Mar 26, 2025
接收日期: May 29, 2025
在线发布日期: Jun 10, 2025
出版日期: Jul 5, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Srivastava, K. and Pandit, B. (2025). Protocol for Generation of Single-Gene Knockout in Hard-to-Transfect THP1 Cell Lines Using CRISPR/Cas9. Bio-protocol 15(13): e5365. DOI: 10.21769/BioProtoc.5365.
分类
分子生物学 > DNA > DNA 重组
免疫学 > 免疫细胞功能
系统生物学 > 基因组学
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link