- EN - English
- CN - 中文
An Alternative Gene Editing Strategy Using a Single AAV Vector
一种使用单一AAV载体的替代性基因编辑策略
发布: 2025年07月05日第15卷第13期 DOI: 10.21769/BioProtoc.5362 浏览次数: 2848
评审: Anonymous reviewer(s)
Abstract
We recently developed an approach for cell type–specific CRISPR/Cas9 editing and transgene expression using a single viral vector. Here, we present a protocol describing how to design and generate plasmids and adeno-associated viruses (AAVs) compatible with this single-vector gene editing approach. This protocol has four components: (1) guide RNA (gRNA) design to target specific genes of interest, (2) ligation and cloning of CRISPR-competent AAV vectors, (3) production of vector-containing AAVs, and (4) viral titer quantification. The resultant vectors are compatible for use with mouse lines expressing the Cas9 protein from Streptococcus pyogenes (SpCas9) and Cre recombinase to enable selective co-expression of standard neuroscience tools in edited cells. This protocol can produce AAVs of any serotype, and the resulting AAVs can be used in the central and peripheral nervous systems. This flexible approach could help identify and test the function of novel genes affecting synaptic transmission, circuit activity, or morphology with a single viral injection.
Key features
• Single-vector CRISPR/Cas9 gene editing and genetically encoded tool delivery for use in mouse central and peripheral nervous systems.
• Can be combined with many genetically encoded tools, including fluorescent proteins, optogenetic and chemogenetic tools, and calcium imaging.
• Requires first-generation cross between a Cre driver mouse line and Cre-dependent SpCas9 mouse line.
• Optimized for use with SpCas9.
Keywords: CRISPR/Cas9 (CRISPR/Cas9)Graphical overview
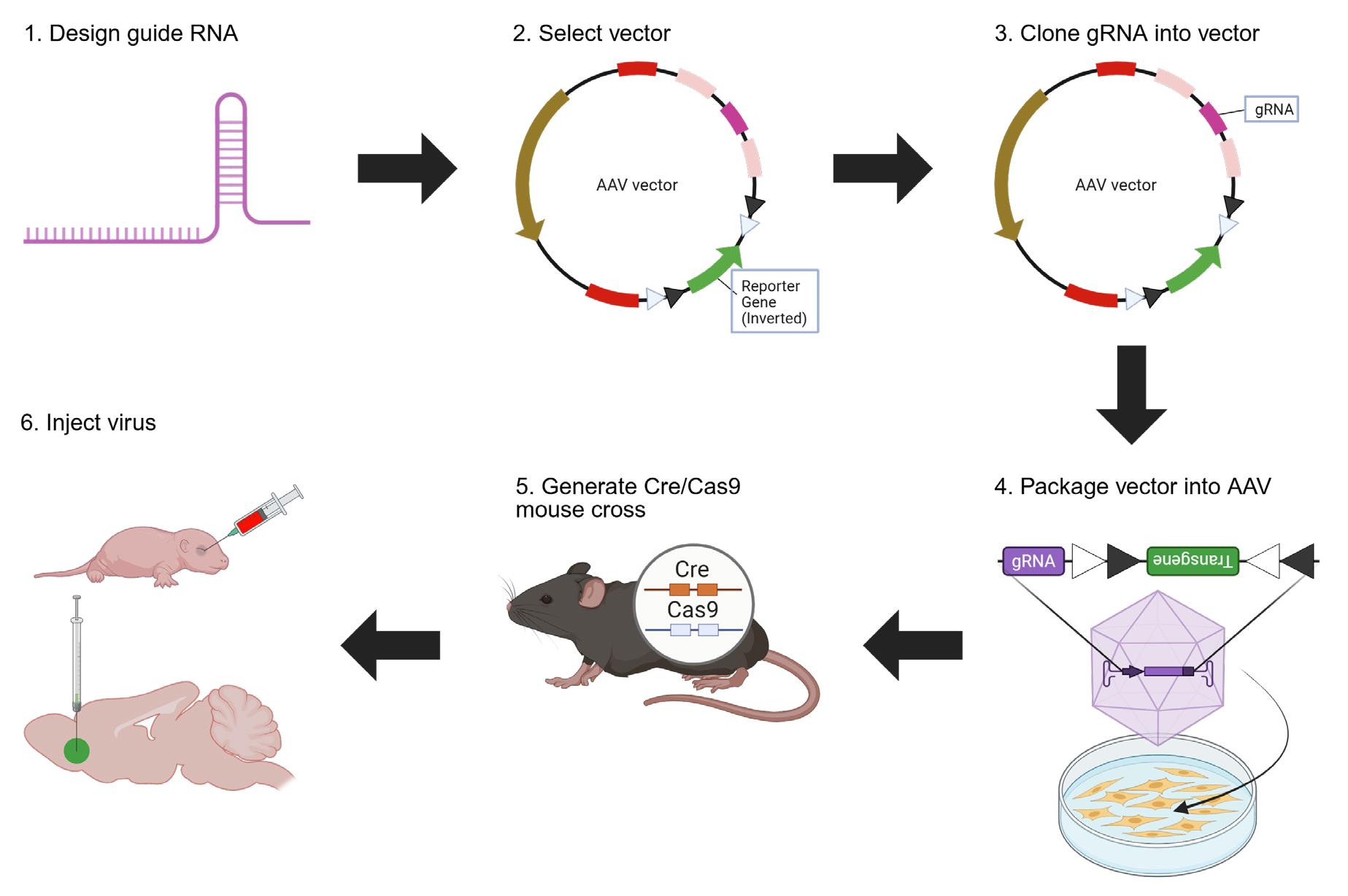
CRISPR vector design and virus production overview. Step 1: Design gRNA against your gene of interest using Ensembl and CRISPOR. Step 2: Select a vector with a spCas9 gRNA scaffold and a reporter gene, such as the pX552 vector (https://www.addgene.org/60958/) or the vectors generated in our original paper (https://www.addgene.org/browse/article/28238669/) (Moffa et al. [1]). Step 3: Digest chosen vector at the gRNA scaffold site, ligate gRNA into the scaffold site, and transform and expand E. coli with the ligated vector. Step 4: Package the vector into the virus in HEK cells and extract the virus from HEK cells. Step 5: Obtain or generate a mouse line expressing spCas9. Step 6: Inject the virus into a mouse line to edit the desired gene and express the reporter gene. Created in https://BioRender.com.
Background
CRISPR/Cas9 gene editing is an effective method to knock out genes in a cell type–specific manner. It uses bacterial Cas9 proteins, which complex with guide RNAs (gRNAs) to target specific DNA regions and cause frame-shifting mutations [2–4]. Successful CRISPR/Cas9 editing has been demonstrated using viral vectors containing gRNA and Cre-dependent Cas9 injected into Cre-driver mice [5–12]. However, this strategy requires the injection of two adeno-associated virus (AAV) vectors to achieve both editing and transgene expression. This is because Cas enzymes are large; only a few can be packaged into AAVs, which have a maximum capacity of ~4.7 kb [8,13–15]. Thus, a second vector carrying a transgene must be co-injected, potentially confounding transduction inefficiencies and limiting applications in the peripheral nervous system (PNS) or difficult-to-target cell types.
In our 2024 paper, we designed and validated an approach to facilitate single-vector CRISPR/Cas9 knockout and genetically encoded tool expression in specific cell types in the peripheral and central nervous systems [1]. Here, we present a protocol describing our approach to facilitate the creation of new viral vectors for diverse downstream applications (Graphical abstract). In section A of our protocol, we describe the process of designing a gRNA against a target gene. In section B, we detail how to insert the chosen gRNA into a CRISPR/spCas9-compatible plasmid, such as those developed by the Zhang lab [12]. Section C is a modified AAV extraction and purification protocol [13] that can be used to produce AAVs of any serotype containing the plasmid generated in B. Finally, in section D, we show one method for AAV quantification via qPCR. Our single-vector approach can be used to screen and test the function of genes for effects on synaptic transmission, circuit activity, or morphology with a single viral injection.
Materials and reagents
Biological materials
1. Mouse strain: Rosa26-LSL-SpCas9-GFP, C57Bl/6 (Jackson Laboratory, catalog number: 0126175) [16]
2. Mouse strain: H11-LSL-SpCas9, C57Bl/6 (Jackson Laboratory, catalog number: 027632) [17]
3. Mouse strain: Vgat-IRES-Cre, C57Bl/6 (Jackson Laboratory, catalog number: 028862) [18]
4. Mouse strain: Vglut2-IRES-Cre, C57Bl/6 (Jackson Laboratory, catalog number: 01693) [18]
5. Mouse strain: PV-IRES-Cre, C57Bl/6 (Jackson Laboratory, catalog number: 017320) [19]
6. Mouse strain: TH-IRES-Cre, C57Bl/6 (MGI, catalog number: 3056580) [20]
7. Plasmid: pX552: pAAV-U6-sgRNA(SapI)-hSyn-GFP-KASH-bGH (Addgene, catalog number: 60958) [12]
8. Plasmid: pAAV-EF1a-DIO-mCherry (Addgene, catalog number: 50462, Bryan Roth)
9. Plasmid: pAAV-EF1α-FLEX-rc[ChRonos-GFP] (Addgene, catalog number: 62725) [21]
10. Plasmid: pGP-AAV-syn-FLEX-jGCaMP8f-WPRE (Addgene, catalog number: 162379) [22]
11. Plasmid: pX552-EF1a-DIO-ChRonos-GFP (with gRNA scaffold) (Addgene, catalog number: 199582) [1]
12. Plasmid: pX552-hsyn-DIO-GCaMP8f (with gRNA scaffold) (Addgene, catalog number: 199580) [1]
13. Plasmid: pX552-EF1a-DIO-mCherry (with gRNA scaffold) (Addgene, catalog number: 199581) [1]
14. Vgat gRNA: 5'-ACCGCTGGGACTTGTTGGACACGG-3' [1]
15. VgatTTT gRNA: 5'-ACCGCTGGGACTTGTTGGACATTT-3' [1]
16. Grin1 gRNA: 5'-ACCGCACAGCAGATGTTCCGCG-3' [1]
17. Grin1TTT gRNA: 5'-ACCGCACGAGCAGATGTTCCTTT-3' [1]
18. Dicer gRNA: 5'-ACCGGACCCATTGGTGAGGAAGCA-3' [1]
19. DicerTTT gRNA: 5'-ACCGGACCCATTGGTGAGGAATTT-3' [1]
20. Stbl3 cells (Invitrogen, catalog number: C737303)
21. AAV 5' ITR Primer: 5'-GGGAAACGCCTGGTATCTTT-3'
22. AAV 3' ITR Primer: 5'-TCTATTGGGAACCAAGCTGG-3'
Reagents
1. SapI Enzyme (New England BioLabs, catalog number: R0569S)
2. CutSmart Buffer (New England BioLabs, catalog number: B6004S)
3. FastAP (Thermo Fisher Scientific, catalog number: EF0651)
4. Agarose (Sigma-Aldrich, catalog number: A0576-100G)
5. SYBR Safe dye (Invitrogen, catalog number: S33102)
6. Takara Gel Purification kit (Takara, catalog number: 740609.50)
7. ATP (New England BioLabs, catalog number: P0756S)
8. 10× PNK T4 buffer (New England BioLabs, catalog number: B0201S)
9. T4 PNK (New England BioLabs, catalog number: M0201L)
10. 2× T7 buffer (New England BioLabs, catalog number: B0318S)
11. T7 DNA ligase (New England BioLabs, catalog number: M0318S)
12. SOC medium (New England BioLabs, catalog number: B9020S)
13. Carbenicillin (Sigma-Aldrich, catalog number: C1389)
14. LB medium (Sigma-Aldrich, catalog number: L3022)
15. QIAprep Spin Miniprep kit (Qiagen, catalog number: 27106)
16. dsDNA Quantification Assay kit (Invitrogen, catalog number: Q32850)
17. Promega Midiprep kit (Promega, catalog number: A7640)
18. HEK 293T cells (ATCC, catalog number: CRL-3216)
19. PEI (Polysciences, catalog number: 239661)
20. OptiPrep/Iodixanol (IOD) (Serumwerk, catalog number: 1893)
21. Benzonase (Sigma-Aldrich, catalog number: E1014)
22. Pluronic detergent (Invitrogen, catalog number: 24040032)
23. DPBS pH 7.0–7.3 (Gibco, catalog number: 14190-136)
24. Takara AAV Titration kit (Takara, catalog number: 6233)
25. Ultrapure distilled water (Invitrogen, catalog number: 10977-023)
26. Tris-Acetate-EDTA (TAE) buffer (EZ Bioresearch, catalog number: S1006)
Solutions
1. SapI digest mix (see Recipes)
2. Oligo phosphorylation mix (see Recipes)
3. Plasmid ligation mix (see Recipes)
4. PEI solution (see Recipes)
5. PEI-DPBS master mix (see Recipes)
6. DNA-DPBS master mix (see Recipes)
7. Lysis buffer (see Recipes)
8. 5 M NaCl (see Recipes)
9. 5× PBS-MK (see Recipes)
10. 15% iodixanol (IOD) (see Recipes)
11. 25% IOD (see Recipes)
12. 40% IOD (see Recipes)
13. 60% IOD (see Recipes)
14. DPBS+Pluronic (see Recipes)
15. AAV lysis solution (see Recipes)
16. 50× AAV primer mix (see Recipes)
17. PCR reaction master mix (see Recipes)
Recipes
1. SapI digest mix
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Empty Vector (e.g., pX552) | 2 μg | x μL |
| 10× CutSmart buffer | 1× | 4 μL |
| SapI enzyme | 0.4 μL | |
| Autoclaved ultrapure distilled water | up to 40 μL | |
| Total | 40 μL |
2. Oligo phosphorylation mix
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Sense gRNA oligo (100 mM stock) | 10 mM | 1 μL |
| Antisense gRNA oligo (100 mM stock) | 10 mM | 1 μL |
| 10× PNK buffer | 1× | 1 μL |
| ATP (25 mM stock) | 1.25 mM | 0.5 μL |
| T4 polynucleotide kinase (PNK) | 1 μL | |
| Autoclaved ultrapure distilled water | 5.5 μL | |
| Total | 10 μL |
3. Plasmid ligation mix
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Digested vector (e.g., pX552) | 20 ng | x μL |
| 1:50 Diluted oligo mix (Recipe 2) | 0.8 μL | |
| 2× T7 buffer | 1× | 5 μL |
| T7 DNA ligase | 0.5 μL | |
| Ultrapure distilled water | bring volume to 10 μL | |
| Total | 10 μL |
4. PEI solution
| Reagent | Quantity or volume |
|---|---|
| PEI | 100 mg |
| Ultrapure distilled water | 310 mL |
Note: Adjust pH to 3 using 12 N HCl.
5. PEI-DPBS master mix
| Reagent | Quantity or volume |
|---|---|
| PEI solution (Recipe 4) | 1,449 μL |
| Ultrapure distilled water | 1,551 μL |
| Total | 3,000 μL |
Note: 3,000 μL of PEI-DPBS master mix is sufficient for preparing one virus using three 15 cm plates. Scale up using the above ratio as needed.
6. DNA-DPBS master mix
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| gRNA vector | 17.11 μg | x μL |
| AAV capsid vector | 68.45 μg | x μL |
| pHelper vector | 34.23 μg | x μL |
| DPBS | bring volume to 3,000 μL | |
| Total | 3,000 μL |
Note: 3,000 μL of DNA-DPBS master mix is sufficient for preparing one virus using three 15 cm plates. Scale up using the above ratios as needed.
7. Lysis buffer
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| NaCl | 150 mM | 0.175 g |
| Tris base | 50 mM | 0.121 g |
| Ultrapure distilled water | 20 mL |
Note: Use 12 N HCl to adjust pH to 8.5.
8. 5 M NaCl
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| NaCl | 5 M | 146.1 g |
| Ultrapure distilled water | 500 mL |
9. 5× PBS-MK
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| MgCl2 (1 M base stock) | 1 mM | 500 μL |
| KCl | 2.5 mM | 93.18 mg |
| 10× PBS | 5× | 250 mL |
| Ultrapure distilled water | 250 mL | |
| Total | 500 mL |
10. 15% iodixanol (IOD)
| Reagent | Quantity or volume |
|---|---|
| IOD | 30 mL |
| 5 M NaCl (Recipe 8) | 24 mL |
| 5× PBS-MK (Recipe 9) | 24 mL |
| Ultrapure distilled water | 42 mL |
| Total | 120 mL |
11. 25% IOD
| Reagent | Quantity or volume |
|---|---|
| IOD | 33 mL |
| 5× PBS-MK (Recipe 9) | 16 mL |
| Ultrapure distilled water | 31 mL |
| Phenol red | 200 μL |
| Total | 80 mL |
12. 40% IOD
| Reagent | Quantity or volume |
|---|---|
| IOD | 80 mL |
| 5× PBS-MK (Recipe 9) | 24 mL |
| Ultrapure distilled water | 16 mL |
| Total | 120 mL |
13. 60% IOD
| Reagent | Quantity or volume |
|---|---|
| IOD | 80 mL |
| Phenol red | 200 μL |
| Total | 80 mL |
14. DPBS+Pluronic
| Reagent | Quantity or volume |
|---|---|
| DPBS | 100 mL |
| Pluronic | 100 μL |
15. AAV lysis solution
| Reagent | Quantity or volume |
|---|---|
| Extracted AAV particles (output of Section C) | 2 μL |
| 10× DNAse I buffer | 2 μL |
| DNAse I | 1 μL |
| Ultrapure distilled water | 15 μL |
| Total | 20 μL |
16. 50× AAV primer mix
| Reagent | Quantity or volume |
|---|---|
| Forward primer | 5 μL |
| Reverse primer | 5 μL |
| Ultrapure distilled water | 15 μL |
| Total | 25 μL |
Note: 25 μL of primer mix will yield 50 qPCR reactions.
17. PCR reaction master mix
| Reagent | Quantity or volume per reaction | Example quantity for 20 reactions |
|---|---|---|
| 2× TB Green | 12.5 μL | 250 μL |
| 50× AAV primer mix (Recipe 16) | 0.5 μL | 10 μL |
| ROX reference dye I/II* | 0.5 μL | 10 μL |
| Ultrapure distilled water | 6.5 μL | 130 μL |
| Total | 20 μL | 400 μL |
Note: Which reference dye you use depends on your qPCR system. See Takara AAVpro Titration Kit v.2 for specific instructions if using this kit.
Laboratory supplies
1. 0.2 mL PCR tubes (Pr1man, catalog number: PR-PCR28ACF)
2. 1.5 mL tubes (Sigma-Aldrich, catalog number: HS4323)
3. Razor blades (VWR, catalog number: 55411-055)
4. 14 mL polystyrene round-bottomed tubes (Falcon, catalog number: 352057)
5. 15 cm tissue culture-treated Petri dishes (Corning, catalog number: 353025)
6. 500 mL bottle top filter (Fisher, catalog number: FB12566510)
7. 15 mL conical tubes (MidSci, catalog number: C15R)
8. OptiSeal ultracentrifuge tubes (Beckman Coulter, catalog number: 361625)
9. 2 mL serological pipettes (Thermo Fisher, catalog number: 170354)
10. Cytiva Vivaspin 20 100 kDa MWCO concentrator tubes (Cytiva, catalog number: 28932363)
11. 0.22 μm filter (TPP, catalog number: 99722)
12. 10 mL syringe (BD, catalog number: 309604)
13. 16 G needles (BD, catalog number: 305197)
14. Low protein binding tubes (Eppendorf, catalog number: 022431064)
Equipment
1. Invitrogen Qubit fluorometer (Invitrogen, catalog number: Q32857)
2. Tissue culture hood
2. Ti-70 rotor
4. Ultracentrifuge capable of speeds of 350,000× g
5. Applied Biosystems 7500 Fast Real-Time PCR System or equivalent real-time PCR system
Software and datasets
1. CRISPOR (https://crispor.tefor.net/crispor.py)
2. Ensembl (https://useast.ensembl.org)
Procedure
文章信息
稿件历史记录
提交日期: Feb 5, 2025
接收日期: May 14, 2025
在线发布日期: Jun 23, 2025
出版日期: Jul 5, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Moffa, J. C., Kalyanaraman, V. and Copits, B. A. (2025). An Alternative Gene Editing Strategy Using a Single AAV Vector. Bio-protocol 15(13): e5362. DOI: 10.21769/BioProtoc.5362.
Moffa, J. C., Bland, I. N., Tooley, J. R., Kalyanaraman, V., Heitmeier, M., Creed, M. C. and Copits, B. A. (2024). Cell-Specific Single Viral Vector CRISPR/Cas9 Editing and Genetically Encoded Tool Delivery in the Central and Peripheral Nervous Systems. eNeuro. 11(7): ENEURO.0438-23.2024. https://doi.org/10.1523/ENEURO.0438-23.2024
分类
神经科学 > 基础技术
微生物学 > 微生物遗传学 > 基因组编辑
分子生物学 > DNA > DNA 重组
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link