- EN - English
- CN - 中文
Activation of X-Succinate Synthases for Fumarate Hydroalkylation Using an In Vitro Activation Method
基于体外激活方法的X-琥珀酸合酶活化及其在富马酸加烷基反应中的应用
(*contributed equally to this work) 发布: 2025年06月20日第15卷第12期 DOI: 10.21769/BioProtoc.5357 浏览次数: 2504
评审: Joana Alexandra Costa ReisAnonymous reviewer(s)
Abstract
X-succinate synthase enzymes (XSSs) are a class of glycyl radical enzymes (GREs) that play a pivotal role in microbial anaerobic hydrocarbon degradation. They catalyze the addition of hydrocarbons to fumarate using a protein-based glycyl radical, which must first be installed by a radical S-adenosylmethionine (rSAM) activating enzyme (AE). Once activated, XSS enzymes can undergo multiple catalytic cycles, forming C(sp3)–C(sp3) bonds with high stereoselectivity—a feature that highlights their potential as asymmetric biocatalysts. Due to the insolubility of XSS-AEs when heterologously expressed in Escherichia coli, studies have relied on in vivo radical installation protocols. Although these methods have illuminated fundamental details of XSS mechanisms, the inability to install a glycyl radical in vitro has limited biochemical studies and biotechnological advances using these enzymes. Here, we describe an in vitro protocol for reconstituting the activity of benzylsuccinate synthase (BSS), an XSS that catalyzes the addition of toluene to fumarate to form R-benzylsuccinate. To enable in vitro glycyl radical installation, we identified a soluble homolog via genome mining: 4-isopropylbenzylsuccinate synthase activating enzyme (IbsAE). IbsAE was expressed in E. coli and anaerobically purified in moderate yields (6–8 mg of protein per liter of culture); herein, we outline the expression and anaerobic purification of both IbsAE and BSS proteins. We describe a reproducible method for in vitro glycyl radical installation using these recombinant proteins and provide guidance on quantifying radical formation. Our optimized protocol consistently achieves 30%–50% radical installation, comparable to other in vitro GRE activations. Lastly, we demonstrate the application of this protocol for in vitro hydroalkylation reactions, achieving high assay yields (89%–97%). This protocol enables biochemical experiments that were previously challenging using cell extracts and accelerated advancements in XSS engineering and use in biocatalysis.
Key features
• Builds upon the method described by Andorfer et al. [1] to thoroughly describe in vitro activation and hydroalkylation using glycyl radical enzymes.
• Useful for studying substrate scope and determining the stereoselectivity of XSS-catalyzed reactions with non-native substrates.
• Can serve as a template for the reconstitution of activity for other XSS enzymes.
• Describes protein production through hydroalkylation steps, which take approximately 6–7 days to complete, given that expressions and purifications are performed in parallel.
Keywords: Glycyl radical enzyme (GRE) (甘氨酰基自由基酶(GRE))Graphical overview
Background
Hydrocarbons, aside from serving as the major feedstock of the chemical industry, are also the primary constituents of natural gas and crude oil. The efficient and selective activation of C–H bonds within these hydrocarbons can be used as a tool for bioremediation as well as a method to convert low-cost hydrocarbon feedstocks into value-added products [2]. X-succinate synthases (XSSs), a class of glycyl radical enzymes (GREs), can catalyze this initial C–H activation of hydrocarbons to stereoselectively hydroalkylate fumarate [3,4]. The development of XSSs as biocatalysts would provide a direct route to access stereochemically complex, sp3-rich molecules from simple, achiral building blocks. Notably, when the native fumarate substrate is used as the olefin substrate, the resulting transformation produces 1,4-dicarbonyls—a privileged motif commonly found in drug scaffolds and valuable building blocks in organic synthesis [5]. Despite their importance, stereocontrolled synthesis of 1,4-dicarbonyls remains a significant challenge [6,7]. Exploring the substrate scope and stereoselectivity of XSS enzymes with non-native substrates, as well as engineering of these enzymes for improved function and expanded reactivity, could unlock powerful new biocatalytic strategies.
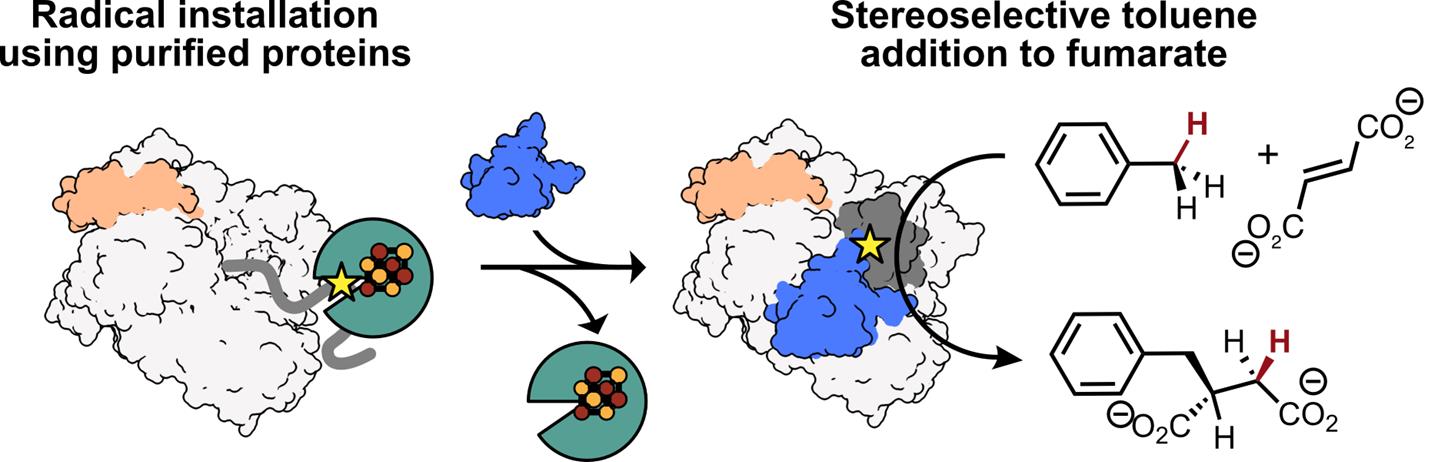
All members of the GRE superfamily feature a 10-stranded β/α-barrel that houses the enzyme’s active site, which contains both a Gly loop and a Cys loop (Figure 1A) [8]. A Gly radical, installed within the Gly loop by a radical S-adenosylmethionine (rSAM) activating enzyme (AE), initiates catalysis by forming a transient thiyl radical on a nearby Cys residue within the Cys loop (Figure 1A, B). This thiyl radical selectively abstracts a hydrogen atom from the benzylic position of toluene, generating a radical species that adds to fumarate via a Giese-type addition. The resulting succinyl radical re-abstracts a hydrogen atom from the catalytic Cys residue, regenerating the thiyl radical and yielding R-benzylsuccinate as the sole product (Figure 1C) [4]. A key advantage of XSS enzymes in biocatalysis is that once this Gly radical is installed, multiple rounds of turnover can occur with no additional cofactors or cosubstrates. While other GRE-AEs have been characterized, producing a soluble XSS-AE for in vitro radical installation using purified proteins remained a challenge until recently [1,8–11].
Here, we describe a method for the in vitro activation of XSS enzymes, specifically focusing on a well-characterized XSS, benzylsuccinate synthase (BSS) [12]. Unlike most GREs, which consist of a single subunit, BSS is composed of three distinct subunits—BSSα, BSSβ, and BSSγ (Figure 1D) [13,14]. We identified a soluble XSS-activating enzyme, 4-isopropylbenzylsuccinate synthase activating enzyme (IbsAE) [15], that can be expressed in E. coli as a soluble protein and demonstrates cross-reactivity with BSS [1]—an uncommon feature among characterized GREs [16]. Through optimization of activation conditions and hydroalkylation assays, we found that the BSSβ subunit inhibits BSS activation (i.e., radical installation) but is essential for hydroalkylation. Because of this finding, we optimized a protocol for producing BSSβ independent of BSSαγ [13], enabling its addition as a reagent in hydroalkylation reactions. We describe heterologous expression and purification protocols for BSSαγ, BSSβ, and IbsAE, which are carried out in an anaerobic chamber due to the oxygen-sensitive [4Fe–4S] clusters present in BSSγ, BSSβ, and IbsAE [13,14]. Recombinantly produced BSSαγ and IbsAE are used to install the Gly radical in BSSα (Figure 1D, 1–2), with radical quantification performed via electron paramagnetic resonance (EPR) spectroscopy. Although SDS-PAGE can also be used to assess radical formation, we discuss the limitations of its use for accurate quantification. Once Gly radical is installed, the addition of BSSβ and substrates to activation reactions enables high assay yields (89%–97%) (Figure 1D, 2–3). As XSSs that catalyze arylalkyl C–H bond activation generally share homologous αβγ subunits, we anticipate that our protocol can be adapted for other arylalkyl-SS enzymes as well. These protocols lay the groundwork for broader application of arylalkyl-SS enzymes in asymmetric biocatalysis. In addition, XSSs exist that activate alkane substrates [17]; however, they typically cluster separately from arylalkyl-SSs and contain additional accessory subunits [18]. We outline potential adaptations to extend our protocol for this distinct class of alkyl-XSSs.
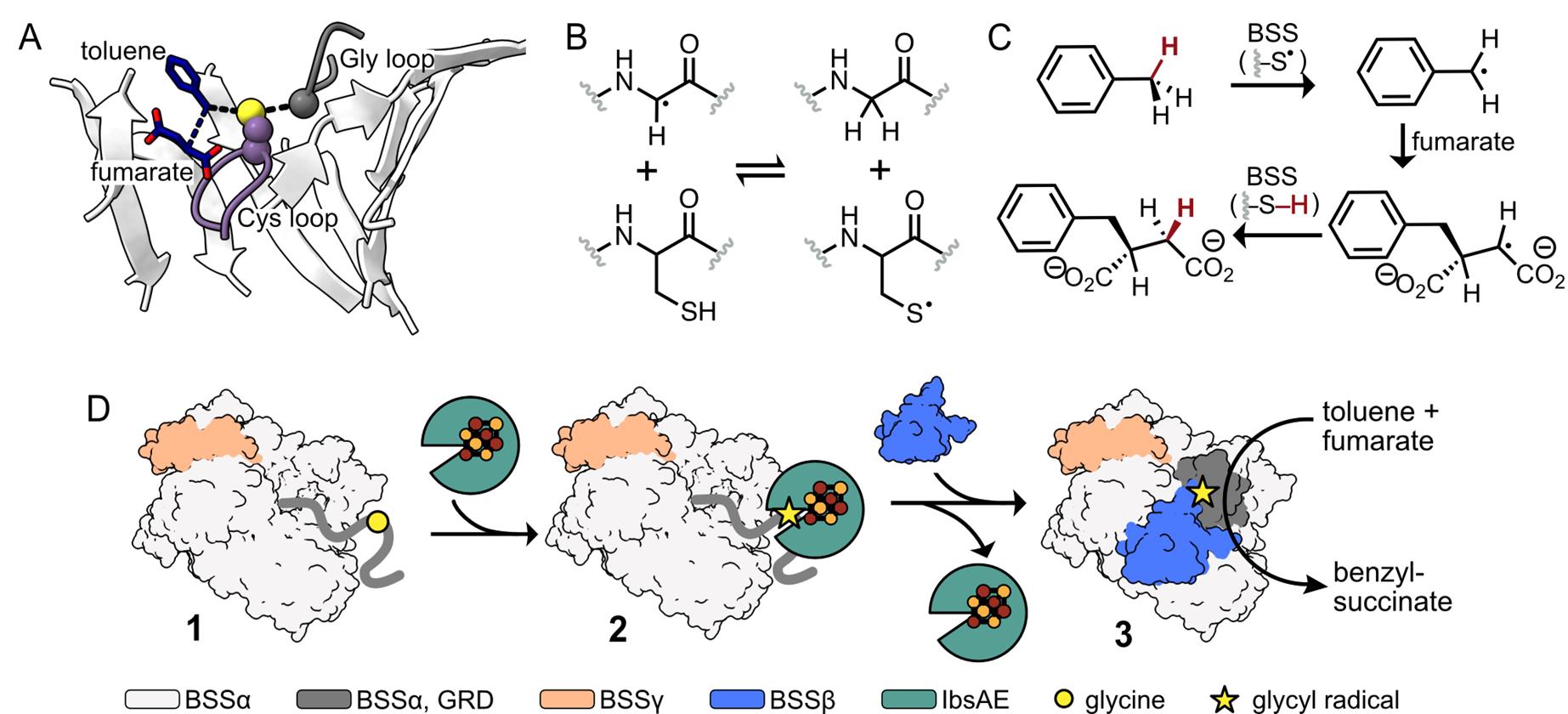
Figure 1. Benzylsuccinate synthase (BSS) catalyzes the addition of toluene to fumarate using amino acid–based radicals. The Gly loop (dark grey) and the Cys loop (purple) of BSS are located in BSS’s 10-stranded β-barrel (white). Toluene (dark blue) is positioned between the catalytic Cys residue and a molecule of fumarate (dark blue). (PDB ID: 5BWE) [11] (B) After installation, the glycyl radical can form a transient thiyl radical on a Cys residue located within the Cys loop. (C) The thiyl radical abstracts an H atom from the methyl group of toluene. The resulting benzylic radical adds to fumarate, generating the succinyl radical, which re-abstracts the H atom from Cys to form R-benzylsuccinate and regenerates the thiyl radical for subsequent catalytic cycles. (D) Cartoon representation of BSS activation and hydroalkylation. 1: BSSαγ adopts an open conformation, making the glycyl radical domain (GRD, dark grey) accessible for binding to IbsAE. 2: When IbsAE (green) is added to reactions, it can bind to the GRD and subsequently install the glycyl radical (shown as a star), activating BSS for catalysis. 3: Upon dilution of the activation reaction and addition of BSSβ, IbsAE is thought to dissociate. BSSβ stabilizes a closed conformation of BSSαγ, which is required for catalysis.
Materials and reagents
Biological materials
1. E. coli NiCo21(DE3) cells (New England BioLabs, catalog number: C2529H)
2. pET-28a-IbsAE. The gene for IbsAE (Uniprot ID A0A096ZNX5) was cloned into pET28a(+) (Novagen, kanamycin resistant) at restriction sites BamHI and HindIII. The N-terminal His-tag was removed, and a C-terminal His-tag was added. The full sequence can be found in Figure S1.
Note: Both N-terminal and C-terminal His-tags produced soluble IbsAE; however, slightly higher yields were obtained when the tag was at the C-terminus.
3. pET-DUET-BSSαγ. The gene for BSSα (Uniprot ID O68395) was cloned into the multiple cloning site 2 (MCS2), and BSSγ (Uniprot ID- O68394) was cloned into the multiple cloning site 1 (MCS1) of pET-DUET-1 (Novagen, ampicillin resistant). A C-terminal His-tag was added to BSSα. BSSγ remained untagged. The full sequence can be found in Figure S2.
4. pRSF-DUET-BSSβ. The gene for BSSβ (Uniprot ID O68396) was cloned into the MCS2 of pRSF-DUET-1 (Novagen, kanamycin resistant). An N-terminal His-tag was added to BSSβ. The full sequence can be found in Figure S3.
Reagents
Reagents for protein expression and purification
1. Lennox broth (LB) (RPI, catalog number: L24060)
2. Terrific broth (TB) (RPI, catalog number: T15000)
3. SOC outgrowth media (New England Biolabs, catalog number: B9020S)
4. Agarose LE (Gold Bio, CAS number: 9012-36-6)
5. Kanamycin sulfate (GoldBio, CAS number: 25389-94-0)
6. Ampicillin (GoldBio, CAS number: 69-52-3)
7. Iron (II) ammonium sulfate hexahydrate [Fe (NH4)2(SO4)2·6H2O] (Sigma-Aldrich, CAS number: 7783-85-9)
8. L-cysteine hydrochloride (Sigma-Aldrich, CAS number: 52-89-1)
9. Isopropyl β-D-1-thiogalactopyranoside (IPTG) (GoldBio, CAS number: 367-93-1)
10. HEPES [4-(2-Hydroxyethyl)piperazine-1-ethanesulfonic acid] (Sigma-Aldrich, CAS number: 7365-45-9)
11. Sodium chloride (NaCl) (Sigma-Aldrich, CAS number: 7647-14-5)
12. Imidazole (Sigma-Aldrich, CAS number: 288-32-4)
13. Glycerol (Fisher chemicals, CAS number: 56-81-5)
14. Lysozyme (Gold Bio, CAS number: 12650-88-3)
15. DNase I, bovine pancreas (Sigma-Aldrich, catalog number: 260913-25MU)
16. TALON® metal affinity resin (Takara Bio, catalog number: 635502)
17. Ni Sepharose Excel (Cytiva, catalog number: 17371201)
18. Precision Plus ProteinTM All Blue standards, protein ladder (Bio-Rad, catalog number: 1610393)
19. 2-Mercaptoethanol (Sigma-Aldrich, CAS number: 60-24-2)
20. Bromophenol blue (Sigma-Aldrich, CAS number: 115-39-9)
21. Sodium dodecylsulfate (SDS) (RPI, CAS number: 151-21-3)
22. Tris(hydroxymethyl) aminomethane HCl (Tris-Cl) (RPI, CAS number: 1185-53-1)
23. 10× Tris/Glycine/SDS buffer (Bio-Rad, catalog number: 1610732)
24. Stain for SDS-PAGE (LC6065 SimplyBlue Safe Stain, catalog number: 465044)
Reagents for radical installation and quantification
1. 5-deazariboflavin (Santa Cruz Biotech., CAS number: 19342-73-5)
2. Dithiothreitol (DTT) (GoldBio, CAS number: 3483-12-3)
3. S-Adenosyl-L-methionine disulfate tosylate (AdoMet or SAM) (Ambeed, CAS number: 97540-22-2)
4. Potassium nitrosodisulfonate (Fremy’s salt) (Sigma-Aldrich, CAS number: 14293-70-0, catalog number: 220930)
Reagents for hydroalkylation reactions and analysis
1. Sodium fumarate dibasic (Sigma-Aldrich, CAS number: 17013-01-3)
2. Toluene (ACS reagent ≥99.5%) (Sigma-Aldrich, CAS number: 108-88-3)
3. Methanol (ACS reagent ≥99.8%) (Sigma-Aldrich, CAS number: 67-51-1)
4. 3-Chlorobenzoic acid (Sigma-Aldrich, CAS number: 535-80-8)
5. 2-Benzylsuccinic acid (Ambeed, CAS number: 884-33-3)
6. Dimethyl sulfoxide (DMSO) (Sigma-Aldrich, CAS number: 67-68-5)
7. LCMS-grade formic acid (Sigma-Aldrich, CAS number: 17013-01-3)
8. HPLC-grade acetonitrile (Sigma-Aldrich, CAS number: 108-88-3)
Solutions
1. LB media (see Recipes)
2. LB/agar media (see Recipes)
3. 50% glycerol stock (see Recipes)
4. TB media (see Recipes)
5. Kanamycin stock (see Recipes)
6. Ampicillin stock (see Recipes)
7. Iron (II) ammonium sulfate hexahydrate stock (see Recipes)
8. L-cysteine hydrochloride stock (see Recipes)
9. 1 M IPTG stock (see Recipes)
10. 1 M HEPES stock (see Recipes)
11. 5 M NaCl stock (see Recipes)
12. 2 M Imidazole stock (see Recipes)
13. Lysis/equilibration buffer (see Recipes)
14. Wash buffer (see Recipes)
15. Elution buffer (see Recipes)
16. Desalting/activation buffer (see Recipes)
17. 1× SDS-PAGE running buffer (see Recipes)
18. 10 mM 5-deazariboflavin stock (see Recipes)
19. 100 mM dithiothreitol (DTT) stock (see Recipes)
20. 36 mM AdoMet stock (see Recipes)
21. Fremy’s salt stock (see Recipes)
22. 100 mM fumarate stock (see Recipes)
23. 2 mM 3-chlorobenzoic acid stock (see Recipes)
24. 20 mM 2-benzylsuccinic acid stock (see Recipes)
25. SDS-PAGE loading dye (see Recipes)
Recipes
1. LB media (1 L)
| Reagent | Final concentration | Amount |
|---|---|---|
| LB | n/a | 20 g |
| Milli-Q Water | n/a | To 1 L |
| Total | 1 L |
After mixing LB and water, autoclave on a standard liquid cycle (25 min sterilization time).
Note: 1 L of media is typically made in a 2.8 L flask, capped with aluminum foil, and then autoclaved. Alternatively, media can be made in autoclavable bottles and autoclaved. In this case, empty flasks should be capped with aluminum foil and autoclaved as well.
2. LB/agar media (25 mL = 1 LB/agar plate)
| Reagent | Final concentration | Amount |
|---|---|---|
| Agar | 1.5% (w/v) | 375 mg |
| LB media | n/a | To 25 mL |
| Antibiotics | Varies with antibiotic | 25 μL |
a. After mixing LB and agar, autoclave on standard liquid cycle (25 min sterilization time). Note that agar will not dissolve until autoclaved.
b. Gently swirl to thoroughly mix. Cool solution to ~50 °C and add appropriate antibiotic. Final concentration (ampicillin = 100 μg/mL; kanamycin = 50 μg/mL).
c. Gently mix the antibiotic into the solution and pour into the plate.
Note: This recipe makes one agar plate; however, more can be made by scaling up the recipe.
3. 50% glycerol stock (1 L)
| Reagent | Final concentration | Amount |
|---|---|---|
| Glycerol | 50% (v/v) | 50 mL |
| Milli-Q Water | n/a | 50 mL |
| Total | 100 mL |
50% glycerol stock is used for making TB, buffers, and glycerol stocks of strains. The stock does not need to be autoclaved if used in TB (Recipe 4) or buffers (Recipes 13–15); however, if used for glycerol stocks of strains, autoclave on standard liquid cycle (25 min sterilization time).
4. TB media (1 L)
| Reagent | Final concentration | Amount |
|---|---|---|
| TB | n/a | 47.6 g |
| 50% glycerol stock | n/a | 8 mL |
| Milli-Q Water | n/a | To 1 L |
| Total | 1 L |
After mixing TB, glycerol, and water, autoclave on a standard liquid cycle (25 min sterilization time).
Note: 1 L of media is typically made in a 2.8 L flask, capped with aluminum foil, and then autoclaved. Alternatively, media can be made in autoclavable bottles and autoclaved. In this case, empty flasks should be capped with aluminum foil and autoclaved as well before use.
5. Kanamycin stock (10 mL)
| Reagent | Final concentration | Amount |
|---|---|---|
| Kanamycin sulfate | 1,000× (50 mg/mL) | 500 mg |
| Milli-Q Water | n/a | To 10 mL |
| Total | 10 mL |
Aliquot as desired and store at -20 °C.
6. Ampicillin stock (10 mL)
| Reagent | Final concentration | Amount |
|---|---|---|
| Ampicillin | 1,000× (100 mg/mL) | 1 g |
| Milli-Q Water | n/a | To 10 mL |
| Total | 10 mL |
Aliquot as desired and store at -20 °C.
7. Iron (II) ammonium sulfate hexahydrate stock (1 mL)
| Reagent | Final concentration | Amount |
|---|---|---|
| Iron (II) ammonium sulfate hexahydrate | 1,000× (75 mg/mL) | 75 mg |
| Milli-Q Water | n/a | To 1 mL |
| Total | 1 mL |
a. Dissolve iron (II) ammonium sulfate hexahydrate in water (vortexing the solution can speed this up).
b. Pass stock through a 0.22 μm syringe filter to sterilize before use.
8. L-cysteine hydrochloride stock (1 mL)
| Reagent | Final concentration | Amount |
|---|---|---|
| L-cysteine hydrochloride | 1,000× (23.5 mg/mL) | 23.5 mg |
| Milli-Q Water | n/a | To 1 mL |
| Total | 1 mL |
a. Dissolve L-cysteine hydrochloride in water (vortexing the solution can speed this up).
b. Pass stock through a 0.22 μm syringe filter to sterilize before use.
9. 1 M IPTG stock (10 mL)
| Reagent | Final concentration | Amount |
|---|---|---|
| IPTG | 1 M | 2.383 g |
| Milli-Q Water | n/a | To 10 mL |
| Total | 10 mL |
Aliquot as desired and store at -20 °C.
10. 1 M HEPES stock, pH 8.0 (1 L)
| Reagent | Final concentration | Amount |
|---|---|---|
| HEPES | 1 M | 238.3 g |
| Milli-Q Water | n/a | To 1 L |
| Total | 1 L |
Adjust the pH to 8.0 by adding NaOH and store at 4 °C.
Note: Dissolve HEPES in approximately 800 mL water, adjust the pH, then add water to reach a final volume of 1 L. The recipe can be scaled down as desired.
11. 5 M NaCl stock (1 L)
| Reagent | Final concentration | Amount |
|---|---|---|
| NaCl | 5 M | 292.2 g |
| Milli-Q Water | n/a | To 1 L |
| Total | 1 L |
Note: The recipe can be scaled down as desired.
12. 2 M imidazole stock, pH 8.0 (1 L)
| Reagent | Final concentration | Amount |
|---|---|---|
| Imidazole | 2 M | 136.2 g |
| Milli-Q Water | n/a | To 1 L |
| Total | 1 L |
Adjust the pH to 8.0 by adding HCl and store at 4 °C.
Note: Dissolve imidazole in approximately 800 mL of water, adjust the pH, then add water to reach a final volume of 1 L. The recipe can be scaled down as desired.
13. Lysis/equilibration buffer (500 mL)
| Reagent | Final concentration | Amount |
|---|---|---|
| 1 M HEPES stock | 50 mM | 25 mL |
| 5 M NaCl stock | 300 mM | 30 mL |
| 50% glycerol stock | 5% | 50 mL |
| Milli-Q Water | n/a | 395 mL |
| Total | 500 mL |
a. Store in a cold room until use.
b. Sparge on a Schlenk line for 20 min with nitrogen gas before bringing into the anaerobic chamber.
Note: This recipe is specifically for use with TALON resin. If using Ni Sepharose resin, imidazole should be added to a final concentration of 10 mM.
14. Wash buffer (500 mL)
| Reagent | Final concentration | Amount |
|---|---|---|
| 1 M HEPES stock | 50 mM | 25 mL |
| 5 M NaCl stock | 300 mM | 30 mL |
| 2 M imidazole stock | 5 mM* | 1.25 mL |
| 50% glycerol stock | 5% | 50 mL |
| Milli-Q Water | n/a | 393.75 mL |
| Total | 500 mL |
a. Store in a cold room until use.
b. Sparge on a Schlenk line for 20 min with nitrogen gas before bringing into the anaerobic chamber.
*Note: This recipe is specifically for use with TALON resin. If using Ni Sepharose resin, the final concentration of imidazole should be 20 mM.
15. Elution buffer (500 mL)
| Reagent | Final concentration | Amount |
|---|---|---|
| 1 M HEPES stock | 50 mM | 25 mL |
| 5 M NaCl stock | 300 mM | 30 mL |
| 2 M imidazole stock | 200 mM* | 50 mL |
| 50% glycerol stock | 5% | 50 mL |
| Milli-Q Water | n/a | 345 mL |
| Total | 500 mL |
a. Store in a cold room until use.
b. Sparge on a Schlenk line for 20 min with nitrogen gas before bringing into the anaerobic chamber.
*Note: This recipe is specifically for use with TALON resin. If using Ni Sepharose resin, the final concentration of imidazole should be 300 mM.
16. Desalting/activation buffer (500 mL)
| Reagent | Final concentration | Amount |
|---|---|---|
| 1 M HEPES stock | 50 mM | 25 mL |
| 5 M NaCl stock | 300 mM | 30 mL |
| Milli-Q Water | n/a | 445 mL |
| Total | 500 mL |
a. Store in a cold room until use.
b. Sparge on a Schlenk line for 20 min with nitrogen gas before bringing into the anaerobic chamber.
17. 1× SDS-PAGE running buffer (1 L)
| Reagent | Final concentration | Amount |
|---|---|---|
| 10× Tris/Glycine/SDS buffer | 1× | 100 mL |
| Milli-Q Water | n/a | 900 mL |
| Total | 1 L |
18. 10 mM 5-deazariboflavin stock (2.66 mL)
| Reagent | Final concentration | Amount |
|---|---|---|
| 5-deazariboflavin | 10 mM | 10 mg |
| DMSO | n/a | To 2.66 mL |
| Total | 2.66 mL |
a. 5-deazariboflavin is light sensitive. Store in 100 μL aliquots in black Eppendorf tubes at -20 °C after freezing in liquid nitrogen. Alternatively, clear tubes can be wrapped in aluminum foil.
b. Transfer an aliquot to the anaerobic chamber in cold beads (-20 °C) prior to activation reactions.
19. 100 mM dithiothreitol stock (DTT) (1 mL)
| Reagent | Final concentration | Amount |
|---|---|---|
| Dithiothreitol | 100 mM | 15.4 mg |
| Desalting/activation buffer | n/a | To 1 mL |
| Total | 1 mL |
a. For desalting/activation buffer, see Recipe 16.
b. Store in 100 μL aliquots in Eppendorf tubes at -20 °C after freezing in liquid nitrogen.
c. Transfer an aliquot to the anaerobic chamber in cold beads (-20 °C) prior to activation reactions.
20. 36 mM AdoMet stock (1 mL)
| Reagent | Final concentration | Amount |
|---|---|---|
| AdoMet | 36 mM | 27.6 mg |
| Desalting/activation buffer | n/a | To 1 mL |
| Total | 1 mL |
a. For desalting/activation buffer, see Recipe 16.
b. Adjust pH with 10 M NaOH until the solution reaches neutral pH as indicated by pH strips.
c. Store in 100 μL aliquots in Eppendorf tubes at -20 °C after freezing in liquid nitrogen.
d. Transfer an aliquot to the anaerobic chamber in cold beads (-20 °C) prior to activation reactions.
21. Fremy’s salt stock
| Reagent | Final concentration | Amount |
|---|---|---|
| Fremy’s salt | X mM | ~3–8 mg |
| Desalting/activation buffer | n/a | To 1 mL |
| Total | 1 mL |
a. For desalting/activation buffer, see Recipe 16.
b. Cycle a bottle of Fremy’s salt into the anaerobic chamber.
c. Add Fremy’s salt to an Eppendorf tube and add activation buffer. The solution should appear pale purple when near the desired final concentration range (~1–3 mM).
d. Transfer a small amount of this solution (20–50 μL) to a clean Eppendorf tube and bring this aliquot out of the anaerobic chamber to find the radical concentration by UV-Vis spectroscopy.
e. Measure the absorbance of the Fremy’s aliquot at 248 nm. The molar absorptivity is 1,690 M-1·cm-1. Use the Beer-Lambert law (A248 = ϵ248bC, where A248 is absorbance at 248 nm, ϵ248 is molar absorptivity at 248 nm, b is the length of the light path, and C is the concentration of the solution) to find the final concentration of Fremy’s radical.
f. The anaerobic Fremy’s stock can then be used to make dilutions for EPR analysis.
Notes:
1. Fremy’s stock is prepared fresh each time a standard curve is made for radical quantification.
2. While buffer conditions used to dissolve Fremy’s salt can vary, buffers with DTT or reductant are not compatible as they quench the radical.
22. 100 mM fumarate stock (1 mL)
| Reagent | Final concentration | Amount |
|---|---|---|
| Sodium fumarate dibasic | 100 mM | 16.0 mg |
| Desalting/activation buffer | n/a | To 1 mL |
| Total | 1 mL |
a. For desalting/activation buffer, see Recipe 16.
b. Store in 100 μL aliquots in Eppendorf tubes at -20 °C after freezing in liquid nitrogen.
c. Transfer an aliquot to the anaerobic chamber in cold beads (-20 °C) prior to activation reactions.
23. 2 mM 3-chlorobenzoic acid stock (10 mL)
| Reagent | Final concentration | Amount |
|---|---|---|
| 3-chlorobenzoic acid | 2 mM | 3.1 mg |
| Desalting/activation buffer | n/a | To 10 mL |
| Total | 10 mL |
a. For desalting/activation buffer, see Recipe 16.
b. Store at -20 °C.
24. 20 mM 2-benzylsuccinic acid stock (10 mL)
| Reagent | Final concentration | Amount |
|---|---|---|
| 2-benzylsuccinic acid | 20 mM | 41.6 mg |
| Desalting/activation buffer | n/a | To 10 mL |
| Total | 10 mL |
a. For desalting/activation Buffer, see Recipe 16.
b. Store at -20 °C.
25. SDS-PAGE loading dye (20 mL)
| Reagent | Final concentration | Amount |
|---|---|---|
| 2-Mercaptoethanol | 5% (v/v) | 500 μL |
| Bromophenol blue | 0.02% (w/v) | 4 mg |
| 50% glycerol | 30% (v/v) | 12 mL |
| SDS | 10% (w/v) | 2 g |
| Tris-Cl | 250 mM | 0.788 g |
| Milli-Q Water | n/a | To 20 mL |
| Total | n/a | 20 mL |
Laboratory supplies
1. 1.7 mL Eppendorf tubes (e.g., Fisher Scientific, catalog number: NC1901967)
2. 15 mL conical tubes (e.g., Avantor, catalog number: 89039-666)
3. 50 mL conical tubes (e.g., Avantor, catalog number: 21008-242)
4. 14 mL sterile culture tubes (e.g., USA Scientific, catalog number: 5618-7262)
5. Standard Petri dish (e.g., USA Scientific, catalog number: 8609-1010)
6. 7.5% Mini-PROTEAN® TGXTM precast protein gel (e.g., Bio-Rad, catalog number: 4561023)
7. 4%–20% Mini-PROTEAN® TGXTM precast protein gel (e.g., Bio-Rad, catalog number: 4561093)
8. 10 mL PierceTM centrifuge columns (e.g., Thermo Scientific, catalog number: 89897)
9. PD-10 desalting column with Sephadex G-25 resin (e.g., Cytiva, catalog number: 17085101)
10. Amicon® Ultra centrifugal filter, 10 kDa MWCO (e.g., Millipore Sigma, catalog number: UFC9010)
11. Amicon® Ultra centrifugal filter, 50 kDa MWCO (e.g., Millipore Sigma, catalog number: UFC9050)
12. 96-well PCR plates (e.g., Alkali Scientific Inc., catalog number: PCFS96)
13. 1,000 μL micropipette tips (e.g., Tip One, catalog number: 1111-2721)
14. 200 μL micropipette tips (e.g., Tip One, catalog number: 1111-0706)
15. 10 μL micropipette tips (e.g., Tip One, catalog number: 1111-3700)
16. 0.22 μm syringe filter (e.g., Fisherbrand, catalog number: 09-720-004)
17. 2,800 mL Fernbach-style culture flask (e.g., Corning Life Sciences, catalog number: 4420-2XL)
18. Alkali Scientific Lab armor metal beads (e.g., Fisher Scientific, catalog number: 50-233-6206)
19. pH strips (e.g., Fisher Scientific, catalog number: M1095350001)
20. 96-well filter plates (Millipore, catalog number: MSGVN2250)
21. Seals for LCMS (BioChromato, catalog number: R80.120.00)
22. Parafilm (e.g., Parafilm M Laboratory Sealing Film, 4-inch × 125-foot roll, catalog number: 164996)
23. EPR tubes (e.g., 4 mm thin wall quartz EPR sample tube 250 mm L, Wilmad, catalog number: 707-SQ-250M)
24. Long-tip glass pipettes for transferring solutions to EPR tubes (e.g., Fisher Scientific, catalog number: 16-800-120)
Equipment
1. Autoclave capable of sterilizing liquid media.
2. Expression equipment:
a. Static incubator for growing LB/agar plates at 37 °C (e.g., Thermo Scientific, catalog number: SHKE6000)
b. Horizonal shaking incubator for growing liquid cultures (e.g., Infors HT Multitron horizontal incubators with cooling, catalog number: MS022TS). Shaker should be able to rotate at 100–250 rpm. Cooling is necessary for expressions at 22 °C. Shaker decks should have clamps to hold 2.8 L Fernbach flasks or adhesive mats (e.g., “Sticky Stuff” from Infors HT, catalog number: MSTRAY)
3. Sonicator to break down the cell pellet inside the Coy (e.g., FisherbrandTM Model 705 Sonic Dismembrator, catalog number: FB705A110)
4. Thermo Sorvall X4 Pro centrifuge for cell pelleting and clarification (e.g., Thermo Scientific, catalog number: 75016052)
5. Small centrifuge to concentrate protein inside the anaerobic chamber (e.g., Eppendorf, model: 5430R, catalog number: 13690005)
6. UV/Vis spectrophotometer to determine concentrations of purified proteins and Fremy’s salt stocks (e.g., Thermo Multiskan Skyhigh microplate spectrophotometer, catalog number: 50-206-8594). Note that low volume measurements (2–10 μL) were performed using a μDrop plate (e.g., Thermo ScientificTM μDrop, catalog number: 50-190-3760)
7. Anaerobic chamber. Two types (described below) were used throughout these studies, yielding consistent and reproducible results in each case:
a. Vinyl anaerobic chamber (e.g., Coy, Type B)
b. Glovebox workstation (e.g., MBraun, Unilab Pro)
8. Block heaters (e.g., VWR, model: Analog Heat Block, catalog number: 12621-104)
9. Freezer (-80 °C) to store protein (e.g., Thermo ScientificTM TSX Series High-Performance Lab Refrigerators, catalog number: TSX50086APM)
10. Freezer (-20 °C) to store small molecule solutions (e.g., Fisher Scientific, model: Isotemp -20 Lab Freezer, catalog number: FBV20FPSA)
11. Water purification system (Thermo Scientific, model: Barnstead GenPure UV/UF-TOC with Bench Top x-CAD Plus, catalog number: 10-451-203)
12. Schlenk line for sparging buffers (ChemGlass, catalog number: AF-0452-01). Note that vacuum attachment is not necessary for these protocols. Nitrogen or argon gas can be used for sparging.
13. Protein electrophoresis equipment (Bio-Rad, catalog number: 1658004)
14. Waters Acquity Premier UPLC–MS system equipped with reversed-phase CORTECS Premier C18+ 1.6 μm 2.1 × 50 mm column
15. Mini-centrifuge (Onilab, model: D1008E)
16. EMX-Plus spectrometer for EPR (Bruker)
Software and datasets
1. SkanIt UV/Vis software (Thermo Scientific)
2. Xenon EPR software (Bruker)
3. Empower LC-MS software (Waters)
Procedure
文章信息
稿件历史记录
提交日期: Mar 19, 2025
接收日期: May 18, 2025
在线发布日期: Jun 5, 2025
出版日期: Jun 20, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/).
如何引用
Vats, A., Anas, S., Chakraborty, A., Liu, J., Ryu, J., Mann, S. M. and Andorfer, M. C. (2025). Activation of X-Succinate Synthases for Fumarate Hydroalkylation Using an In Vitro Activation Method. Bio-protocol 15(12): e5357. DOI: 10.21769/BioProtoc.5357.
分类
生物化学 > 蛋白质 > 活性
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link