- EN - English
- CN - 中文
An Optimized Ex Vivo Protocol for Quantitative Electrophysiological Assessment of Neuromuscular Junctions and Skeletal Muscle Function Using the Aurora System
基于Aurora系统的离体神经肌肉接头与骨骼肌功能定量电生理评估优化方案
(#Contributed equally to this work §Technical contact: cancui@cuhk.edu.hk) 发布: 2025年06月20日第15卷第12期 DOI: 10.21769/BioProtoc.5353 浏览次数: 1937
评审: Olga KopachVolodymyr KrotovAnonymous reviewer(s)
Abstract
The neuromuscular junction (NMJ) is critical for muscle function, and its dysfunction underlies conditions such as sarcopenia and motor neuron diseases. Current protocols for assessing NMJ function often lack standardized stimulation parameters, limiting reproducibility. This study presents an optimized ex vivo method to evaluate skeletal muscle and NMJ function using the Aurora Scientific system, incorporating validated stimulation protocols for both nerve and muscle to ensure consistency. Key steps include tissue preparation in a low-calcium, high-magnesium solution to preserve NMJ integrity, determination of optimal muscle length, and sequential stimulation protocols to quantify neurotransmission failure and intratetanic fatigue. By integrating rigorous standardization, this approach enhances reproducibility and precision, providing a robust framework for investigating NMJ pathophysiology in aging and disease models.
Key features
• Dual stimulation modes enable direct muscle and indirect nerve stimulation to isolate NMJ-specific dysfunction.
• Optimized stimulation parameters for nerve (5 mA, 0.8 ms pulse width) and muscle (300 mA, 0.2 ms pulse width) on mouse model.
• Preservation of NMJ integrity through dissection in low-calcium, high-magnesium artificial cerebrospinal fluid (aCSF) and synthetic interstitial fluid (SIF).
• Quantitative analysis of neurotransmission failure and intratetanic fatigue using standardized equations.
Keywords: Ex vivo muscle function (离体肌肉功能)Graphical overview
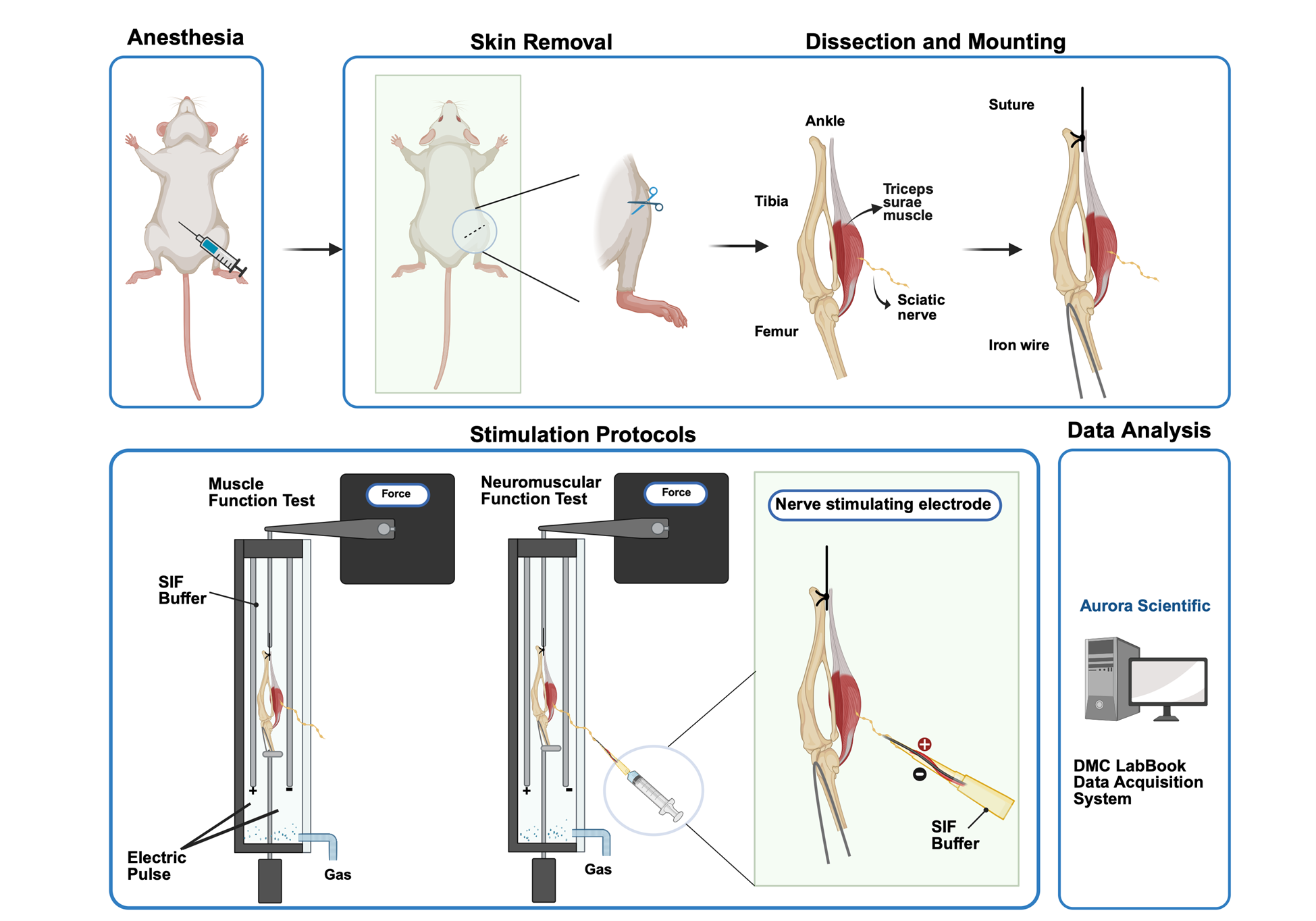
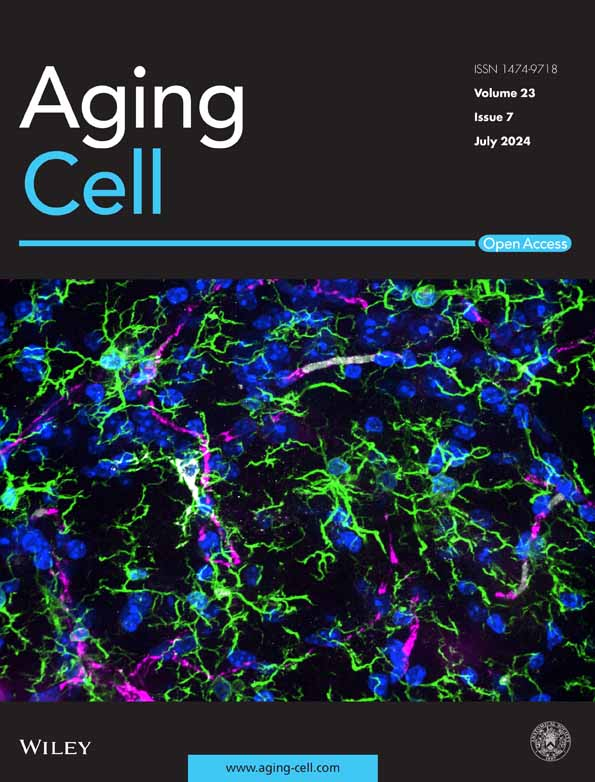
Steps to stimulate triceps surae–sciatic nerve to test muscle and neuromuscular junction (NMJ) function contraction: For tissue preparation, the triceps surae–sciatic nerve is dissected in a low-calcium, high-magnesium artificial cerebrospinal fluid (aCSF). Then, the preparation is transferred to the ex vivo system tank in synthetic interstitial fluid (SIF) perfused with mixed oxygen (95% oxygen, 5% carbon dioxide). Tendons are clamped to a force transducer (Aurora Scientific 800C-1, Newmarket, Canada) to measure isometric contractions. During stimulation, two parallel electrodes stimulate muscle contraction directly. The suction electrode induces muscle contraction indirectly by stimulating the sciatic nerve. Optimal muscle length (L0) is determined by incrementally stretching the muscle until maximal twitch force is achieved, ensuring standardized sarcomere alignment. Twitch force (F0) and tetanic force (Ft) of muscle and nerve are acquired by stimulating the muscle and sciatic nerve in a validated sequence. Results are recorded with the DMC LabBook data acquisition system (Aurora Scientific) and analyzed by the analysis system.
Background
The neuromuscular junction (NMJ), a specialized synapse bridging motor neurons and skeletal muscle fibers, is indispensable for voluntary movement and autonomic function. Its dysfunction underlies a spectrum of pathologies, including sarcopenia, amyotrophic lateral sclerosis (ALS), and myasthenic syndromes, which affect millions globally [1–3]. Traditional methodologies for assessing NMJ integrity and function have relied on in vivo electrophysiology, histological analyses, and electron microscopy (EM), which provide critical insights into synaptic ultrastructure [4,5]. Ex vivo systems, such as the Aurora Scientific apparatus, have emerged as powerful tools for isolating NMJ physiology by enabling direct muscle and indirect nerve stimulation [6]. Despite their utility, existing protocols exhibit critical limitations, notably inconsistent stimulation parameters (e.g., pulse width, frequency) and inadequate preservation of NMJ structure during tissue preparation, leading to interlaboratory variability and diminished reproducibility [7].
Recent studies employing ex vivo systems have underscored the importance of standardized parameters in quantifying neurotransmission failure (NF) and intratetanic fatigue (IF), key metrics of NMJ resilience. For instance, Rizzuto et al. (2015) demonstrated that precise stimulation protocols are essential for detecting NMJ dysfunction in the SOD1(G93A) mouse model of amyotrophic lateral sclerosis (ALS), highlighting how variable pulse widths and frequencies can obscure synaptic deficits [7]. Similarly, Pratt et al. (2015) reported that inconsistent stimulation currents in dystrophic (mdx) mice led to misinterpretations of NMJ fatigue, emphasizing the need for protocol standardization [8]. Moreover, the use of non-physiological dissection buffers often compromises NMJ morphology [9]. Franco et al. (2014) revealed that conventional saline solutions accelerate synaptic vesicle depletion, compromising NMJ integrity [10]. This issue is particularly relevant in aging models, where NMJ morphology is already vulnerable. Recent work by Wang et al. (2023) further demonstrated that low-calcium, high-magnesium dissection buffers mitigate synaptic transmission inhibition, preserving NMJ architecture in murine sarcopenia models [11]. These technical shortcomings could impede translational research, leading to conflicting outcomes in therapeutic trials for neuromuscular disorders [12,13].
To address these challenges, this protocol introduces an optimized ex vivo framework that integrates (1) validated stimulation parameters for nerve (5 mA, 0.8 ms pulse width) and muscle (300 mA, 0.2 ms pulse width) to maximize activation fidelity; (2) a low-calcium, high-magnesium dissection buffer to preserve NMJ ultrastructure by inhibiting spontaneous neurotransmitter release [10]; and (3) standardized equations for quantifying NF and IF, enabling direct comparisons across experimental models. By coupling these advancements with the Aurora Scientific system’s dual-mode lever and real-time data acquisition, this approach enhances precision in evaluating NMJ pathophysiology, as demonstrated in murine models of sarcopenia [14–16]. The protocol’s reproducibility and translational relevance are further evidenced by its application in identifying NMJ-specific deficits in aging mice [17].
This methodological advancement bridges a critical gap in neuromuscular research and provides a platform for mechanistic studies of NMJ-targeted therapies. It could accelerate the development of interventions for conditions where synaptic failure precedes muscle atrophy, such as early-stage ALS or chemotherapy-induced neuropathy in the murine model [18].
Materials and reagents
Biological materials
1. C57BL/6, senescence accelerated mouse (SAMP8) and the age-matched control senescence-resistant inbred strains (SAMR1)
Reagents
1. Sodium pentobarbital (Alfasan, catalog number: 16582)
2. Sodium chloride (NaCl) (Thermofisher, catalog number: S/3160/65)
3. Potassium chloride (KCl) (Thermofisher, catalog number: P/4240/60)
4. Magnesium sulfate, heptahydrate (MgSO4·7H2O) (Sigma-Aldrich, catalog number: M1880)
5. Sodium bicarbonate (NaHCO3) (Thermofisher, catalog number: 424270010)
6. HEPES (VWR, catalog number: 0511-250G)
7. Glucose (Sigma-Aldrich, catalog number: G8270)
8. Calcium chloride (CaCl2) (Sigma-Aldrich, catalog number: 902179-1KG)
9. Sodium dihydrogen phosphate monohydrate (NaH2PO4. H2O) (AlfaAesarTM, catalog number: 115911)
10. Sodium gluconate (NaC6H11O7) (ShangHai EKEAR Bio@Tech Co. LTD, catalog number: EC208-407-7)
11. Sucrose (Thermofisher, catalog number: BP220-1)
12. Potassium dihydrogen phosphate (KH2PO4) (VWR, catalog number: 26936.293)
Solutions
1. 1× Synthetic interstitial fluid (SIF) (see Recipes)
2. 1 × Artificial cerebrospinal fluid (aCSF) (see Recipes)
Recipes
1. 1× Synthetic interstitial fluid (SIF), pH 7.4 ± 0.05
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| NaCl | 123 mM | 7.188 g |
| KCl | 3.5 mM | 0.261 g |
| MgSO4·7H2O | 0.7 mM | 0.173 g |
| HEPES | 10 mM | 2.383 g |
| Glucose | 5.5 mM | 0.991 g |
| CaCl2 | 2 mM | 0.222 g |
| NaH2PO4. H2O | 1.7 mM | 0.235 g |
| NaC6H11O7 | 9.5 mM | 1.160 g |
| Sucrose | 7.5 mM | 2.567 g |
| Total (optional) | n/a | 1,000 mL |
2. 1× Artificial cerebrospinal fluid (aCSF), pH 7.4 ± 0.05
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| NaCl | 128 mM | 7.480 g |
| KCl | 1.9 mM | 0.142 g |
| MgSO4·7H2O | 6.5 mM | 1.602 g |
| NaHCO3 | 26 mM | 2.184 g |
| Glucose | 10 mM | 1.802 g |
| CaCl2 | 0.85 mM | 0.094 g |
| KH2PO4 | 1.2 mM | 0.163 g |
| Total (optional) | n/a | 1,000 mL |
Laboratory supplies
1. 5 mL disposable syringe (Terumo, catalog number: SS+10L)
2. 3-way stopcock (Taobao, Shengdakang company, catalog number: n/a)
3. Single strand ultra-fine soft high-temperature silver-plated wire (0.3 mm) (Taobao, Shanghai Haoyu tech company, catalog number: n/a)
4. Iron wire (0.3 mm) (Taobao, catalog number: n/a)
5. Mersilk braided silk suture 4-0 (Johnson & Johnson, catalog number: SA83G)
6. BNC to dual alligator clip oscilloscope probe test lead cable (1 m, 3 ft) (Taobao, catalog number: n/a)
7. 200 μL pipette tips (Multi, catalog number: 15730)
8. 90 × 20 mm cell culture dish (SPL Life Sciences, catalog number: 20100)
Equipment
A. Surgical tools (Figure 1)
1. Serrated forceps
2. Tian Gong ultra-fine microsurgical forceps (0.34 mm) (Taobao, catalog number: n/a)
3. Tian Gong microdissecting spring scissors (1.1 cm) (Taobao, catalog number: n/a)
4. Tian Gong premium gold-handled scissors (9.5cm) (Taobao, catalog number: ZHTG)
5. Metal robotic arm (Taobao, catalog number: n/a)
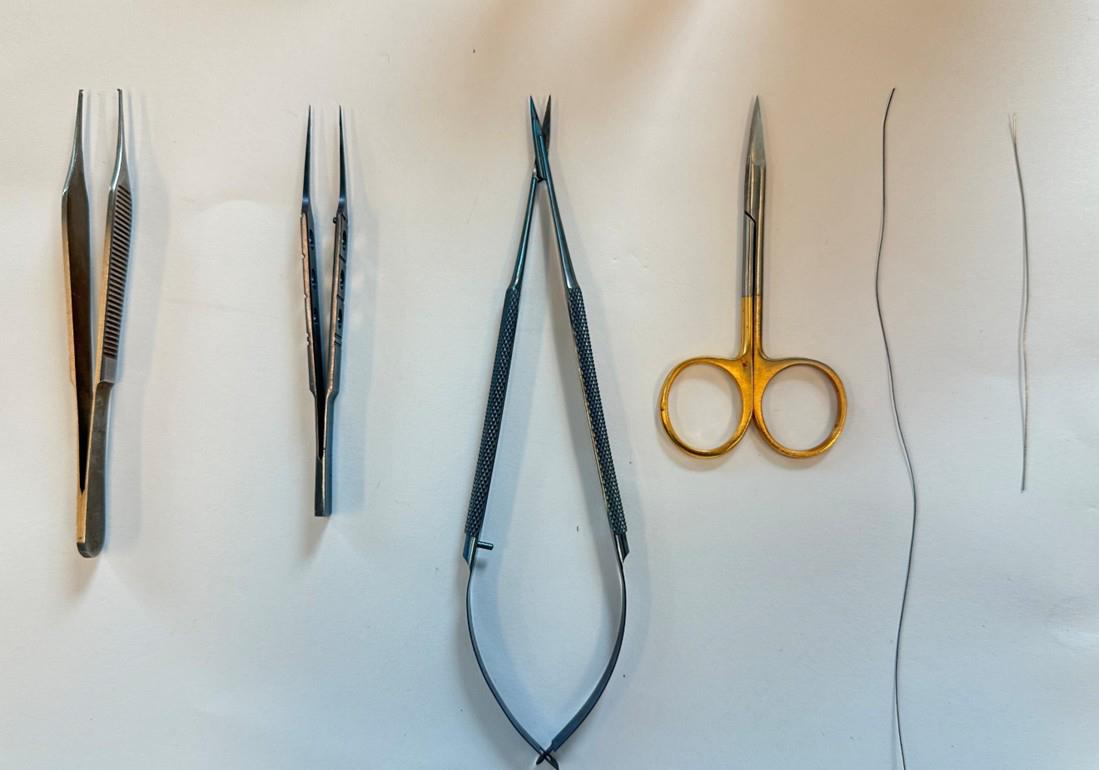
Figure 1. Surgical tools for sample preparation. From left to right, serrated forceps, ultra-fine microsurgical forceps, microdissecting spring scissors, gold-handled scissors, suture and wire are prepared for animal experiment.
B. Testing and recording equipment (Figure 2)
1. In-vitro Test Apparatus (Vertical, Aurora Scientific Inc., Newmarket, Canada, catalog number: 800C-1)
2. Dual-mode lever, stimulator (Aurora Scientific Inc., Newmarket, Canada, catalog number: 150A)
3. 5% CO2/O2 in compressed gas cylinder (Linde HKD Ltd)

Figure 2. Ex vivo muscle functional test system. The system consists of the Dual-mode lever stimulator (left) and the In-vitro Test Apparatus (right).
Software and datasets
1. DMC LabBook data acquisition system (Aurora Scientific Inc, Newmarket, Canada, catalog number: 610A, version DMCV6.000)
2. Analysis system (Aurora Scientific Inc, Newmarket, Canada, catalog number: 615B, version DMAv5.501)
Procedure
文章信息
稿件历史记录
提交日期: Mar 17, 2025
接收日期: May 22, 2025
在线发布日期: Jun 10, 2025
出版日期: Jun 20, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Cui, C., Bao, Z., Chow, S. K., Wang, Q., Chai, S., Xu, Z., Jiang, Q. and Cheung, W. H. (2025). An Optimized Ex Vivo Protocol for Quantitative Electrophysiological Assessment of Neuromuscular Junctions and Skeletal Muscle Function Using the Aurora System. Bio-protocol 15(12): e5353. DOI: 10.21769/BioProtoc.5353.
分类
神经科学 > 神经解剖学和神经环路 > 坐骨神经
细胞生物学 > 组织分析 > 电生理学
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link