- EN - English
- CN - 中文
An Optimized RNA Extraction Method From Micro-quantities of Guinea Pig Cartilage and Synovium for Osteoarthritis Research
用于骨关节炎研究的微量豚鼠软骨和滑膜RNA优化提取方法
发布: 2025年06月20日第15卷第12期 DOI: 10.21769/BioProtoc.5348 浏览次数: 1601
评审: Anonymous reviewer(s)
Abstract
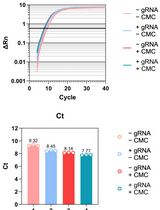
Osteoarthritis (OA) is the primary cause of joint impairment, particularly in the knee. The prevalence of OA has significantly increased, with knee OA being a major contributor whose pathogenesis remains unknown. Articular cartilage and the synovium play critical roles in OA, but extracting high-quality RNA from these tissues is challenging because of the high extracellular matrix content and low cellularity. This study aimed to identify the most suitable RNA isolation method for obtaining high-quality RNA from microquantities of guinea pig cartilage and synovial tissues, a relevant model for idiopathic OA. We compared the traditional TRIzol® method with modifications to spin column–based methods (TRIspin-TRIzol®/RNeasyTM, RNeasyTM kit, RNAqueousTM kit, and Quick-RNATM Miniprep Plus kit), and an optimized RNA isolation protocol was developed to increase RNA yield and purity. The procedure involved meticulous sample collection, specialized tissue processing, and measures to minimize RNA degradation. RNA quality was assessed via spectrophotometry and RT–qPCR. The results demonstrated that among the tested methods, the Quick-RNATM Miniprep Plus kit with proteinase K treatment yielded the highest RNA purity, with A260:280 ratios ranging from 1.9 to 2.0 and A260:230 ratios between 1.6 and 2.0, indicating minimal to no salt contamination and RNA concentrations up to 240 ng/μL from ⁓20 mg of tissue. The preparation, storage, homogenization process, and choice of RNA isolation method are all critical factors in obtaining high-purity RNA from guinea pig cartilage and synovial tissues. Our developed protocol significantly enhances RNA quality and purity from micro-quantities of tissue, making it particularly effective for RTqPCR in resource-limited settings. Further refinements can potentially increase RNA yield and purity, but this protocol facilitates accurate gene expression analyses, contributing to a better understanding of OA pathogenesis and the development of therapeutic strategies.
Key features
• Enables efficient RNA isolation from small, individual cartilage samples, eliminating the need for pooling and requiring minimal laboratory equipment.
• Provides a reliable and cost-effective method for obtaining high-quality RNA suitable for RT–qPCR and gene expression analysis, from challenging tissue types like cartilage.
Keywords: Cartilage (软骨)Graphical overview
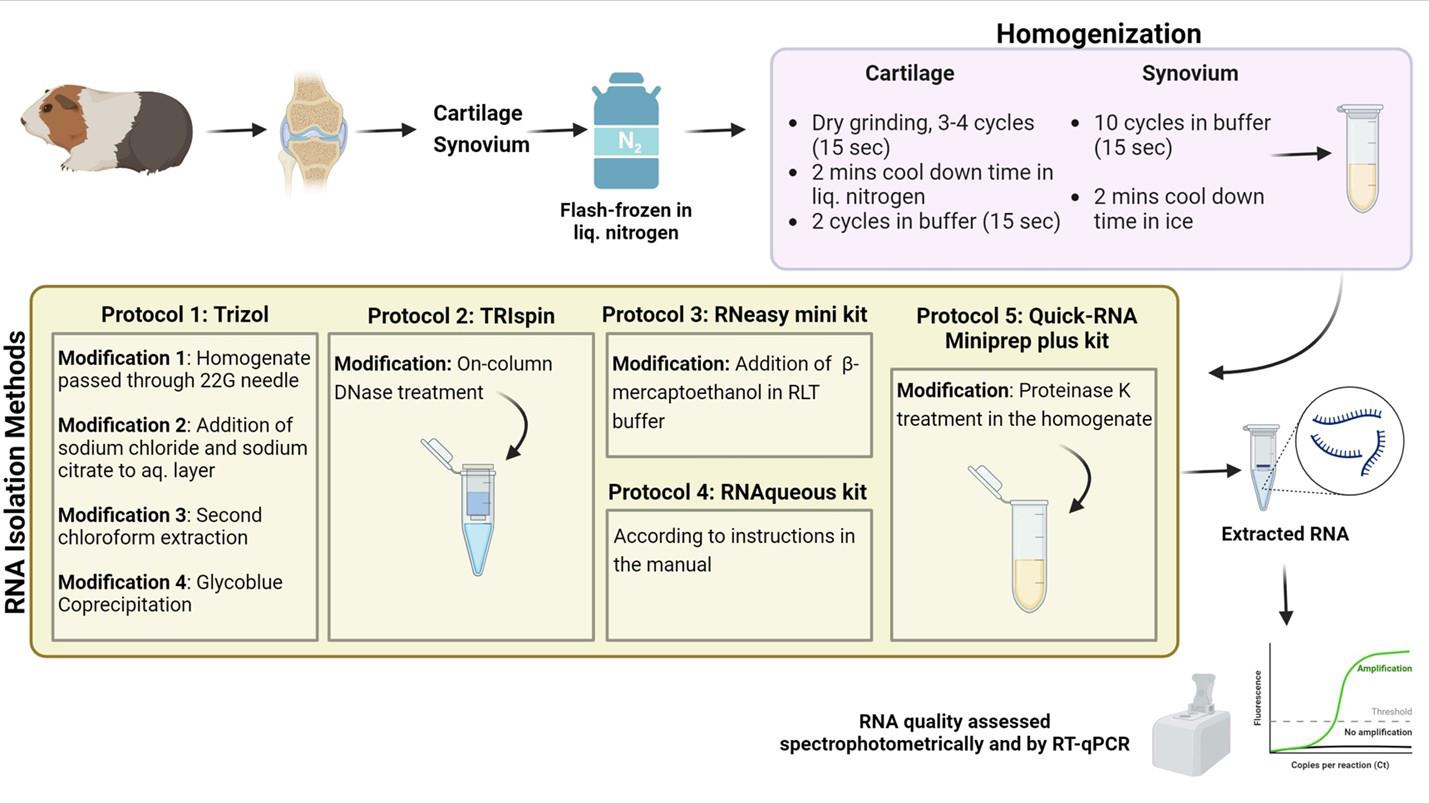
Graphical representation comparing modified RNA isolation protocols for efficient RNA extraction from guinea pig cartilage and synovium
Background
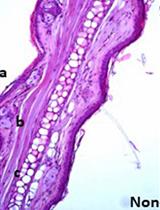
Osteoarthritis (OA) is the prevailing cause of joint impairment, particularly in the knee joint, and is characterized primarily by inflammation, redness in the affected joints, persistent joint pain, and restricted movement. Based on the Global Burden of Disease Study 2019, research from 1990 to 2019 revealed a substantial increase in the prevalence of OA in specific joints. The prevalence of OA cases increased significantly by 113%, increasing from 247 million in 1990 to 527 million in 2019, with knee OA playing a major role in contributing to the overall burden [1]. Therefore, there is a compelling need to gain deeper insights into the pathological mechanisms underlying OA, and novel approaches, methodologies, and techniques must be employed to elucidate the pathogenesis of OA and to further explore therapeutic strategies. Articular cartilage, which is the most affected tissue in osteoarthritic conditions and undergoes degenerative disease, has a distinctive structural composition and consists of a very sparse distribution of chondrocytes surrounded by copious amounts of extracellular matrix (ECM) [2]. The majority of articular cartilage is composed of ECM, which consists mainly of glycosaminoglycans, collagens, and proteoglycans [3]. Moreover, the gradual deterioration of the articular surfaces in OA results in a significant decrease in the amount of cartilage and, consequently, in the amount of available tissue. This adds to the existing challenges faced while isolating RNA from cartilage tissues and underscores the importance of devising an appropriate methodology for procuring high-quality RNA from such tissues. In addition, articular cartilage is relatively hard, making it even more difficult to homogenize for RNA extraction without specialized equipment. To date, some of the reported methods for isolating RNA from articular cartilage are suitable only for larger cartilage samples weighing at least 100 mg, or they require the use of freezer-mills or microdisembranators [4,5] for homogenization, which are not readily available in all laboratories. The technique of RNA extraction from cartilage by first isolating chondrocytes [6,7] has certain drawbacks, as it can lead to the loss of heterogeneous chondrocyte populations in the cartilage and can be stressful for the cells, leading to a skewed representation of the gene expression profile. In addition, this is also a time-consuming process, especially when dealing with a large number of samples, which can lead to delays in downstream analysis. Protocols for the isolation of RNA from the cartilage of humans or large animals (e.g., chickens and horses) [8,9] are available, but this is not the case for Dunkin–Hartley guinea pigs, despite being the most appropriate model for studying idiopathic osteoarthritis [10–12]. While studies in smaller species such as mice often achieve high RNA quality, they frequently require pooling cartilage tissue from multiple animals to obtain sufficient material for extraction [13]. Moreover, advanced techniques such as single-cell RNA sequencing in mice also necessitate pooling cartilage from a minimum of five mice [14], which may not be feasible or align with certain experimental designs, especially in contexts where animal availability is restricted.
Owing to its limited cellular population and elevated proteoglycan content, the extraction of high-quality total RNA from cartilage poses a particular challenge. This makes gene expression analysis directly from the cartilage tissues technically demanding, yet critically important. High-quality RNA is essential not only for accurate gene expression profiling but also for exploring epigenetic mechanisms involved in OA, such as regulation by non-coding RNAs (e.g., microRNAs). Gene expression analysis provides a molecular snapshot of the disease state and allows for the identification of differentially expressed genes and signaling pathways involved in OA progression, inflammation, and cartilage degradation. These molecular signatures can help uncover key regulatory nodes that drive disease onset and advancement, thereby serving as targets for therapeutic intervention. While gene expression alone may not fully elucidate OA pathogenesis, it remains a foundational and indispensable tool in dissecting the molecular mechanisms of the disease and guiding the development of targeted, mechanism-based therapies.
Based on the importance of investigating gene expression within chondrocytes, the challenges associated with RNA isolation from small amounts of cartilage tissue, and the limitations of existing methodologies, we present an improved RNA isolation protocol in resource-limited setups from the articular cartilage and synovium of Dunkin–Hartley guinea pigs. The protocol has been designed to optimize sample collection and tissue processing procedures, aiming to minimize RNA degradation while increasing RNA quality. It has significant implications for advancing both basic OA research and translational applications. Our findings demonstrate that the RNA obtained from the articular cartilage and synovial tissues of Dunkin–Hartley guinea pigs using the optimized methodology is suitable for downstream applications such as RTqPCR. However, a few limitations remain. While the extracted RNA exhibited acceptable purity and concentration, the RNA integrity number (RIN) values were below the threshold generally required for high-throughput applications such as RNA sequencing or transcriptome profiling. Despite this, the RNA quality was sufficient for reliable RT–qPCR analysis, providing a practical and cost-effective alternative, particularly for resource-limited settings. Additionally, we observed variability in RNA yield from 12-month-old animals, likely due to increased OA severity and the corresponding reduction in the quantity of available tissue. This variability poses a challenge for reproducibility and consistency. Future refinements in tissue handling, preservation, and extraction techniques may be necessary to further enhance RNA quality and expand the applicability of this protocol to broader transcriptomic studies.
Materials and reagents
Reagents
1. Liquid nitrogen
2. TRIzol® reagent (Invitrogen, catalog number: 15596026 or equivalent)
3. RNaseZap or RNase AWAY for RNase decontamination
4. Chloroform (Himedia, catalog number: AS040 or equivalent)
5. GlycoblueTM coprecipitant (Invitrogen, catalog number: AM9516)
6. Isopropanol (Himedia, catalog number: AS068 or equivalent)
7. Ethanol (Himedia, catalog number: MB106 or equivalent)
8. Nuclease-free water (Sigma, catalog number: 3098 or equivalent)
9. RNeasyTM Mini kit (Qiagen, catalog number: 74104)
10. RNAqueousTM kit (Invitrogen, catalog number: AM1912)
11. Quick-RNATM Miniprep Plus kit (Zymo Research, catalog number: R1057)
12. RNase-free DNase set (Qiagen, catalog number: 79254)
13. miRCURY LNA RT kit (Qiagen, catalog number: 339340)
14. miRCURY LNA SYBR Green PCR kit (Qiagen, catalog number: 339346)
15. Custom miRCURY LNA 5S rRNA PCR assay (Qiagen, catalog number: 339317, GeneGlobe ID: YCP0527201)
16. iScript cDNA Synthesis kit (Bio-Rad, catalog number: 1708891)
17. β-actin primers (Sigma-Aldrich, Merck, purification: desalt)
18. Trisodium citrate dihydrate (Himedia, catalog number: TC249)
19. Sodium chloride (Himedia, catalog number: MB023)
Solutions
1. 3 M sodium citrate (see Recipes)
2. 3 M sodium chloride (see Recipes)
3. 70% ethanol (see Recipes)
Recipes
1. 3 M sodium citrate
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Trisodium citrate dihydrate (MW = 294.10 g/mol) | 3 M | 44.12 g (for 50 mL final volume) |
| Nuclease-free water | N/A | 50 mL |
Weigh 44.12g of trisodium citrate dihydrate and dissolve it in approximately 40mL of nuclease-free water. Stir the solution until completely dissolved, then adjust the final volume to 50mL with nuclease-free water. Store the solution at 4 °C.
2. 3 M sodium chloride
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Sodium chloride (MW = 58.44 g/mol) | 3 M | 3.51 g (for 50 mL final volume) |
| Nuclease-free water | N/A | 50 mL |
Weigh 3.51g of sodium chloride and dissolve it in approximately 40mL of nuclease-free water. Stir the solution until completely dissolved, then adjust the final volume to 50mL with nuclease-free water. Store the solution at 4 °C.
3. 70% ethanol
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Absolute ethanol (100%) | 70% (v/v) | 35 mL |
| Nuclease-free water | 30% (v/v) | 15 mL |
Laboratory supplies
1. Stainless-steel vials (Biospec, catalog number: 2007)
2. 3.2 mm stainless-steel beads (Biospec, catalog number: 11079132ss)
3. 1.5 mL microcentrifuge tubes (Eppendorf tubes 3810X, catalog number: 0030125215)
4. 22G needles (Lifelong Safeway) and 1 mL syringe (Accu-Shot One, catalog number: 1A1R1P)
Equipment
1. Mini-Beadbeater-16 (Biospec, catalog number: 607)
2. Centrifuge with a temperature range of up to 4 °C (ThermoFisher Scientific, catalog number: 75007213)
3. Micro-pipettes 0.5–10, 10–100, 20–200, and 100–1,000 μL (Eppendorf)
4. Benchtop centrifuge (ThermoFisher Scientific, catalog number: 75002414)
5. Laminar hood (Labtop Laminar Air Flow, product ID: 5116877-50624733960) or a clean and isolated working area
6. NanoDrop®-ND 1000 spectrophotometer (ThermoFisher Scientific, catalog number: ND-1000)
7. RT-qPCR (Bio-Rad, model: CFX96)
Software and datasets
1. NanoDrop® ND-1000 Software (v3.7.1, Thermo Fisher Scientific, 2015)
2. Bio-Rad CFX Manager Software (v3.1, Bio-Rad Laboratories, 2014)
3. Microsoft Excel
Procedure
文章信息
稿件历史记录
提交日期: Apr 17, 2025
接收日期: May 18, 2025
在线发布日期: May 30, 2025
出版日期: Jun 20, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/).
如何引用
Bhardwaj, N., Rana, D. and Kaur, J. (2025). An Optimized RNA Extraction Method From Micro-quantities of Guinea Pig Cartilage and Synovium for Osteoarthritis Research. Bio-protocol 15(12): e5348. DOI: 10.21769/BioProtoc.5348.
分类
分子生物学 > RNA > RNA 提取
细胞生物学 > 组织分析
医学 > 发炎
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link