- EN - English
- CN - 中文
Surface Plasmon Resonance for the Interaction of Capsular Polysaccharide (CPS) With KpACE
利用表面等离子体共振技术检测莢膜多糖(CPS)与KpACE的相互作用
发布: 2025年06月20日第15卷第12期 DOI: 10.21769/BioProtoc.5346 浏览次数: 3552
评审: Anonymous reviewer(s)
Abstract
The study of carbohydrate–protein interactions is crucial for clarifying biological processes and identifying potential drug candidates. However, due to the complex structure of carbohydrates, such as high molecular weight, dynamic flexibility, and high solution viscosity, it is challenging to study their interactions with diverse proteins. Conventional analytical techniques like isothermal titration calorimetry (ITC), X-ray crystallography, molecular dynamics (MD) simulations, and nuclear magnetic resonance (NMR) spectroscopy have limitations in revealing these molecular interactions. Surface plasmon resonance (SPR), an advanced optical biosensor technique, overcomes these limitations. It enables real-time, label-free monitoring of the interaction dynamics between carbohydrates and proteins through a continuous flow over a chip surface. In this study, we utilized SPR-based techniques to explore the interaction of capsular polysaccharides (CPS) of Klebsiella pneumoniae and the enzyme KpACE (K. pneumoniae acetylated capsule esterase). Our SPR-based analytical platform has several advantages, including shorter experimental time, a simulated physiological state, and minimal sample requirements for investigating carbohydrate–protein interactions. This approach expands the applicability scope of SPR technology and provides a valuable tool for a wide range of research. By using SPR, we successfully verified that KpACE acts on the acetyl groups of CPS, demonstrating its enzymatic activity, which is crucial for understanding the pathogenic mechanism of K. pneumoniae and developing potential antibacterial drugs.
Key features
• Conduct rapid screening of carbohydrate–protein interactions to determine binding affinity (KD).
• Perform a comprehensive binding assay to assess the interactions between capsular polysaccharides (CPS) and mutant enzymes, thereby validating their catalytic sites.
• Apply the methodology to achieve a highly sensitive, label-free, simulated physiological environment for substrate–enzyme interaction studies.
Keywords: Surface plasmon resonance (表面等离子体共振)Graphical overview
Background
Severe infections caused by hypervirulent strains of Klebsiella pneumoniae have a high mortality rate. Capsular polysaccharides (CPS) play a decisive role in determining the virulence of K. pneumoniae. CPS are a class of carbohydrates with distinctive structural features [1–3]. They consist of various monosaccharide residues, diverse glycosidic linkages, α/β-anomer center, and various chemical modifications [4,5]. For example, O-acetylation modification exists on the CPS of K1 and K2 hypervirulent K. pneumoniae [6,7]. However, the regulatory mechanism of the acetylation level remains unclear due to the lack of experimental evidence. K. pneumoniae acetylated capsule esterase (KpACE) is a novel acetyl esterase. Identifying its natural substrate is key to clarifying the molecular mechanism of KpACE [8]. Understanding how KpACE interacts with CPS can help us understand the role of acetylation modification in the virulence of K. pneumoniae, which may provide new ideas for developing drugs against K. pneumoniae infections.
Current methods for studying carbohydrate–protein binding are diverse, each with its own principles, advantages, and limitations. Isothermal titration calorimetry (ITC) is an early-stage technique for analyzing molecular interactions, especially useful in the study of lectin-carbohydrate interactions [9,10]. It measures the thermal energy exchange during molecular interactions to directly obtain thermodynamic parameters such as binding constants, stoichiometry, enthalpy changes (ΔH), and entropy changes (ΔS) [11,12]. However, this method requires a large sample size, is insensitive to weak binding interactions, and has low thermal effects.
Nuclear magnetic resonance (NMR) spectroscopy can provide information about the spatial proximity of atoms and clarify carbohydrate–protein binding sites and conformational changes by analyzing chemical shift variations and nuclear Overhauser effect (NOE) interactions [13]. Nevertheless, when analyzing high-molecular-weight carbohydrates (over 50 kDa), NMR spectroscopy exhibits reduced sensitivity and often requires isotopic labeling, which increases the complexity and duration of the experiment [14].
X-ray crystallography and cryo-electron microscopy (Cryo-EM) are pivotal for elucidating the high-resolution structures of glycoprotein complexes. They can identify binding sites, interaction modes, glycosylation sites, and binding interfaces [15]. For instance, Cryo-EM has helped reveal the dynamic conformational changes of the SARS-CoV-2 spike glycoprotein during viral membrane fusion. However, these techniques require high sample quality [16]. X-ray crystallography depends on the formation of well-ordered and high-quality crystals, which is difficult, especially for conformationally flexible carbohydrates [17,18]. Similarly, Cryo-EM requires samples to be rapidly frozen to maintain their native state, imposing strict requirements on sample stability and purity [19].
Fluorescence spectroscopy (FSS) can screen the interactions between carbohydrates and proteins by detecting fluorescence polarization or energy transfer efficiency. FSS measurements and fluorescence resonance energy transfer (FRET) have been used to study the interaction of DNA-bound EcoRI DNA methyltransferase (M.EcoRI) and obtain binding constants and thermodynamic parameters [20]. Carbohydrate microarrays combine surface-immobilized glycan libraries with high-resolution analytical platforms such as high-throughput scanning and fluorescent staining systems [21,22]. However, fluorescent labeling may interfere with the natural binding dynamics, resulting in apparent rather than intrinsic binding parameters, and the experimental setups often face challenges in reproducibility and success rates. In addition, GROMACS-based MD simulations have been used to model the interaction between low-density lipoprotein receptor (LDLR) glycoproteins and alphavirus envelope proteins to verify the impact of key residue mutations on binding affinity [23]. However, computational methods have limitations such as force field inaccuracy and oversimplified hydration models, which often lead to energy deviations from experimental measurements, especially for flexible carbohydrates with complex conformational landscapes.
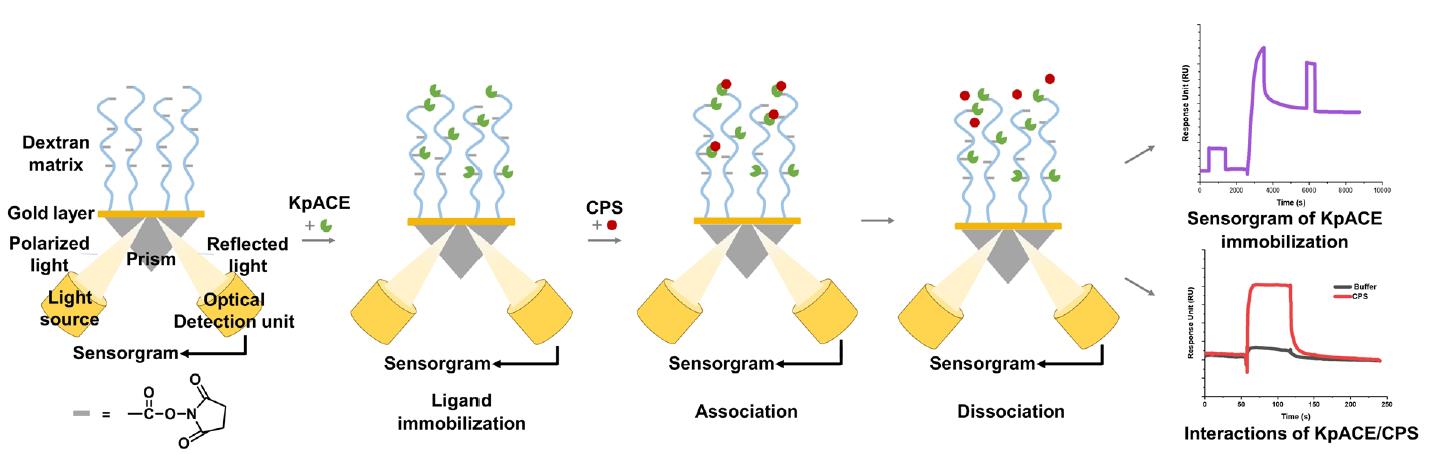
Surface plasmon resonance (SPR) is an advanced biosensing technique that can quantify molecular binding dynamics in real-time. Ligands are immobilized on a biosensor chip, and analytes in solution flow over the surface [24]. Binding events cause changes in the refractive index near the sensor surface, which are detected as shifts in the resonance angle, enabling precise measurement of association/dissociation rates without the need for labeling. SPR allows for high-sensitivity detection (down to pM-level binding affinities) and supports the comparative analysis of binding kinetics (e.g., association/dissociation rates) and thermodynamics (e.g., equilibrium constants) of structurally related compounds [25]. The most prominent limitation lies in the intrinsic structural complexity of proteins. For membrane proteins that require preservation of their native conformation, such as G protein–coupled receptors (GPCRs) or ion channels, maintaining functional integrity during SPR analysis remains a substantial challenge, thereby impeding accurate characterization of protein–ligand interactions. In the limited number of reported cases, a common strategy involves engineering short peptide tags (e.g., His-tags) as immobilization sites to minimize structural disruption. For example, the GPCR was immobilized via a His-tagged system, where a specific anti-His10 antibody was pre-coupled to the sensor chip surface for protein capture [26]. However, this approach significantly increases experimental complexity and failure rates due to issues with tag accessibility and antibody compatibility. Another critical challenge arises from high-refractive-index buffer systems containing glycerol or DMSO, which introduce significant signal interference. Although dynamic background subtraction can partially mitigate this issue, experiments become infeasible beyond threshold concentrations of these additives. A further unavoidable constraint is the prohibitively high cost of SPR instrumentation [27]. Acquisition and maintenance expenses often exceed the budgetary capacity of small laboratories, limiting broader access to this technology.
Despite these limitations, SPR remains a highly versatile tool for investigating molecular interactions. Its key advantages include broad applicability to structurally simple proteins, label-free detection, real-time kinetic monitoring, and minimal sample consumption. SPR also possesses inherent potential for high-throughput screening: under conditions that preserve protein activity, sequential injection of multiple analytes can be achieved. However, for scenarios involving diverse protein–analyte combinations, the implementation of high-throughput workflows requires either multiple sensor chips or advanced instrument configurations that facilitate the parallel immobilization of tens to hundreds of proteins on a single chip. Its real-time and high-throughput interaction data make it an essential tool for screening high-affinity binding sites in bioactive molecules [28]. In the exploration of interactions between sugars and proteins, this technology has seen broad and successful applications [29–31].
In this study, we employed an SPR-based assay to screen high-efficiency mutants of KpACE and explore its interaction with CPS. This not only helps to identify the enzymatic catalytic site of KpACE but also provides critical insights into potential therapeutic targets to mitigate bacterial virulence. The findings of this study underscore the importance of SPR in revealing the molecular mechanisms of enzyme–CPS interactions and contribute to drug discovery and development.
Materials and reagents
Biological materials
1. Recombinant proteins KpACE and its mutants (KpACEH180A and KpACEH370A) with a 6×His tag at the N-terminus (proteins were constructed in our laboratory using standard expression and purification techniques [8])
2. Klebsiella pneumoniae capsular polysaccharides (CPS) (carbohydrates were prepared in our laboratory using standard extraction and purification techniques [8])
Reagents
1. Amine Coupling kit [1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide hydrochloride (EDC), N-hydroxysuccinimide (NHS), and 1.0 M ethanolamine-HCl pH 8.5 (EtA)] (Global Life Sciences Solutions, Cytiva, catalog number: BR100050)
2. PBS-P+ buffer 10× (Global Life Sciences Solutions, Cytiva, catalog number: 28995084)
3. Glycine 1.5 (Global Life Sciences Solutions, Cytiva, catalog number: BR100354)
4. 10 mM acetate 4.0, acetate 4.5, acetate 5.0, and acetate 5.5 (Global Life Sciences Solutions, Cytiva, catalog numbers: BR100349, BR100350, BR100351, BR100352)
5. NaOH 50 mM (Global Life Sciences Solutions, Cytiva, catalog number: BR100358)
Solutions
1. PBS-P+ running buffer (see Recipes)
2. Protein solution, 200 μg/mL (see Recipes)
3. Carbohydrate solution, 1 mg/mL (see Recipes)
Recipes
1. PBS-P+ running buffer
Add 25 mL of the PBS-P+ buffer 10× to 225 mL of double-distilled water (ddH2O) in a 1,000 mL InfinityLab solvent bottle.
2. Protein solution, 200 μg/mL
Recombinant KpACE and its mutants were quantified by measuring the absorbance at 280 nm using a NanoDrop spectrophotometer. After the calculation was completed, the proteins were diluted to 200 μg/mL with PBS-P+ running buffer to prepare 200 μL.
3. Carbohydrate solution
Prepare CPS at 1 mg/mL (2.22 × 106 Da): Add 1 mg of the CPS to 1 mL of the PBS-P+ buffer.
Laboratory supplies
1. Pipettes (Rainin Pipet-Lite XLS+ 20, 200, 1,000 μL) (Merrler Toledo, catalog numbers: 17014392, 17014391, 17014382)
2. Pipette tips (Rainin RC LTS 10, 200, 1,000 μL) (Merrler Toledo, catalog numbers: 17014340, 17014341, 17014342)
3. 96-well polystyrene microplates (Global Life Sciences Solutions, Cytiva, catalog number: BR100503)
4. Microplate foil, 96-well (Global Life Sciences Solutions, Cytiva, catalog number: 28975816)
5. Rubber caps, type 2 (Global Life Sciences Solutions, Cytiva, catalog number: BR100411)
6. Series S Sensor Chip CM5 (Global Life Sciences Solutions, Cytiva, catalog number: BR100530)
7. InfinityLab solvent bottle, clear, 1,000 mL (Agilent, catalog number: 9301-6524)
8. Microcentrifuge tube 1.5 mL (Biosharp, catalog number: BS-15-M)
Equipment
1. Biacore T200 SPR (Global Life Sciences Solutions, Cytiva, catalog number: 28975001)
Software and datasets
1. Biacore T200 Control Software
2. Biacore T200 Analysis Software (Evaluation Software 3.1)
3. OriginPro Learning Edition (https://www.originlab.com/OriginProLearning.aspx)
Procedure
文章信息
稿件历史记录
提交日期: Mar 16, 2025
接收日期: May 6, 2025
在线发布日期: Jun 5, 2025
出版日期: Jun 20, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Wang, Z., Wang, L., Zhang, X., Zhang, J. R. and Cai, C. (2025). Surface Plasmon Resonance for the Interaction of Capsular Polysaccharide (CPS) With KpACE. Bio-protocol 15(12): e5346. DOI: 10.21769/BioProtoc.5346.
分类
生物化学 > 糖类 > 多糖
微生物学 > 微生物生物化学 > 糖类
生物物理学
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link