- EN - English
- CN - 中文
Cryopreservation of Bulk-Produced Primary Rat Oligodendrocyte Progenitor Cells
大规模制备的大鼠少突胶质前体细胞的冻存方法
(§Technical contact: hanki313@ajou.ac.kr) 发布: 2025年06月20日第15卷第12期 DOI: 10.21769/BioProtoc.5345 浏览次数: 1409
评审: Anonymous reviewer(s)
Abstract
Primary oligodendrocyte cultures are a crucial driving force for in vitro research on oligodendrocytes (OLs) and myelin. Various methods are available to obtain oligodendrocyte lineage cells, primarily from neonatal rodent brains or human induced pluripotent stem cells (iPSCs). In this protocol, we describe a step-by-step procedure for detaching and cryopreserving primary rat oligodendrocyte progenitor cells (OPCs), followed by the thawing, proliferation, and differentiation of the cryopreserved OPCs. After freezing in a serum-free cryopreservation medium, the OPCs can be preserved at -80 °C for up to two months without notable changes in viability, proliferation, or differentiation into mature OLs. Cryopreserved OPCs can be differentiated into mature OLs with robust myelin processes and the capacity to wrap around neuron-mimicking structures. Combined with the author’s method for primary OL culture, which allows for bulk production of OPCs, OPC cryopreservation may substantially improve the efficiency of in vitro OL research.
Key features
• This protocol recommends the use of a specific culture method that enables the simple, bulk production of primary rat OPCs.
• Through this protocol, researchers may obtain large numbers of cryopreserved OPCs, which can be reserved for up to two months.
• This protocol facilitates the planning of in vitro experiments and reduces the effort required to maintain adequate numbers of primary OPCs for large-scale experiments.
Keywords: Oligodendrocyte progenitor cell (OPC) (少突胶质前体细胞(OPC))Graphical overview
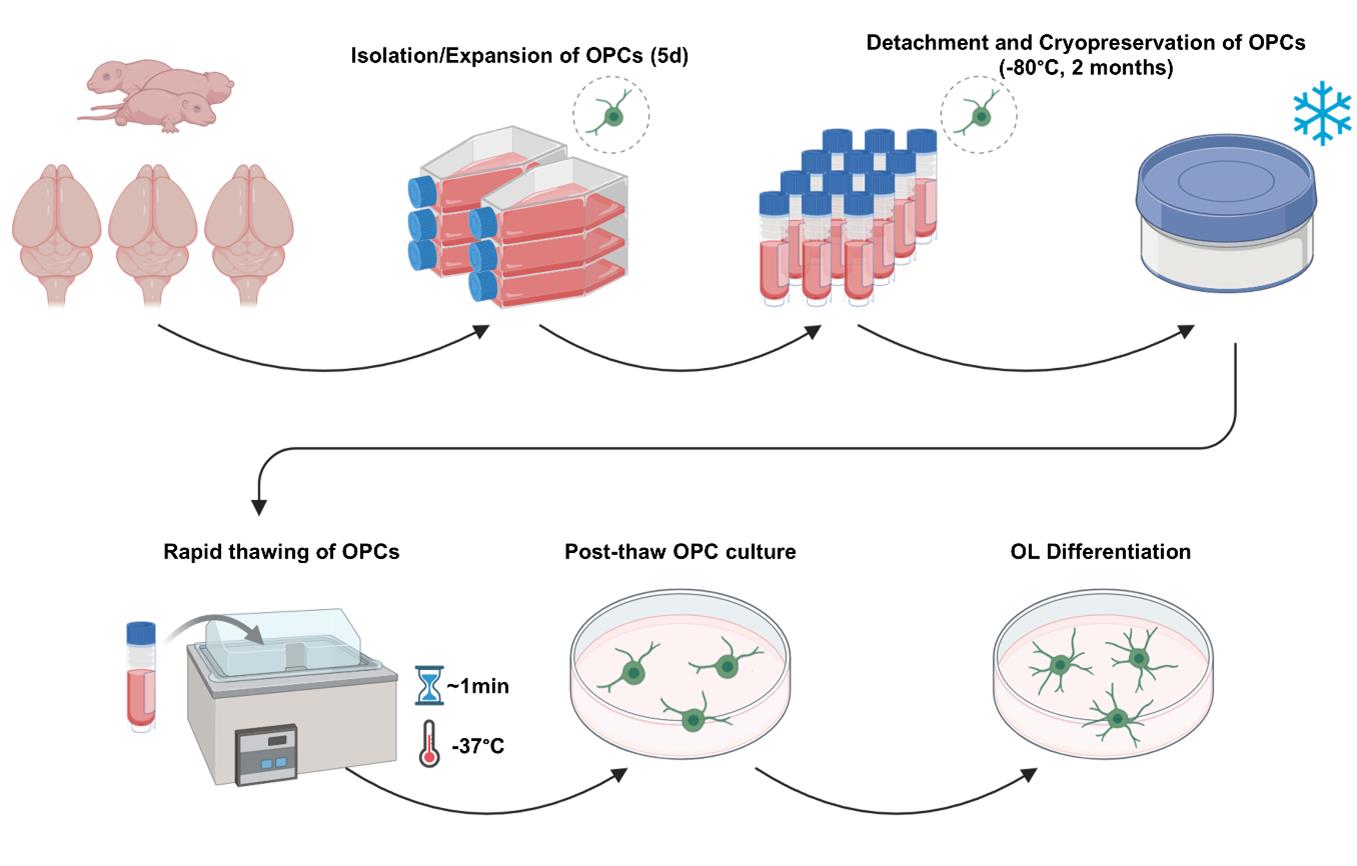
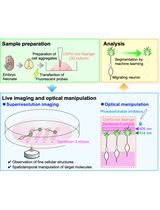
Procedure for the bulk generation, cryopreservation, thawing, and post-thaw culture of primary rat oligodendrocyte progenitor cells (OPCs)
Background
Oligodendrocytes (OLs) are the myelinating glial cells in the central nervous system (CNS). The myelination, saltatory conduction, and metabolic support that OLs provide are vital to normal CNS function [1–3]. The deterioration of OLs and myelin ultimately leads to neurodegeneration, which is implicated in numerous CNS disorders such as multiple sclerosis, ischemic brain disease, neurodegenerative diseases, or traumatic brain injury [4–8].
In vitro, primary OL cultures are effective for observing various biological processes of the OL lineage, including oligodendrocyte progenitor cell (OPC) proliferation, migration, differentiation into myelinating OLs, and myelination. Primary OL cultures derived from either neonatal rodent brains or human induced pluripotent stem cells (hIPSCs) are primarily used for in vitro OL research [9–13]. However, despite the advantage of accurately reproducing OL function, primary OL cultures are generally not an efficient research model due to their lengthy culture periods, low cell yield, and high material and technical requirements.
In this protocol, we outline a step-by-step process for cryopreserving OPCs derived in bulk from neonatal rat brains. Cryopreserved OPCs can be maintained for up to two months without compromising viability, proliferative capacity, and differentiation into myelinating OLs [14]. Combined with an efficient, high-yield primary OL culture method we have recently developed, researchers may obtain large numbers of OPC reserves, which may reduce the effort needed to constantly generate and maintain primary OL cultures and facilitate in vitro OL research.
Materials and reagents
Biological materials
1. Sprague-Dawley rats (postnatal Day 1, wild type, of either sex) (Orient Bio)
Reagents
1. Distilled water (DW) (autoclaved, sterile)
2. 70% ethanol (in distilled water)
3. Isopropanol
4. Poly-D-lysine (PDL) (Sigma-Aldrich, catalog number: P6407)
5. OptiprepTM density gradient medium (Sigma-Aldrich, catalog number: D1556)
6. Phosphate-buffered saline (PBS), no calcium, no magnesium (Cytiva, catalog number: SH30256.01)
7. AccumaxTM (Merck Millipore, catalog number: SCR006)
8. Papain suspension (Worthington, catalog number: LS003126)
9. CELLBANKER® 2 (Zenogen Pharma)
10. Hank’s balanced salt solution (HBSS), no calcium, no magnesium (Gibco, catalog number: 14175-095)
11. Hibernate-A medium (Thermo Fisher Scientific, catalog number: A1247501)
12. Dulbecco’s modified Eagle’s medium/Ham’s F12 (DMEM/F12) (Thermo Fisher Scientific, catalog number: 11320033)
13. Neurobasal medium (NBM) (Thermo Fisher Scientific, catalog number: 21103049)
14. Penicillin/streptomycin (Cytiva, catalog number: SV30010)
15. GlutaMAXTM (Thermo Fisher Scientific, catalog number: 35050-061)
16. B27 supplement (Thermo Fisher Scientific, catalog number: 17504-044)
17. N2 supplement (Thermo Fisher Scientific, catalog number: 17502048)
18. Platelet-derived growth factor-AA (PDGF-AA) (Peprotech, catalog number: 100-13A)
19. Basic fibroblast growth factor (bFGF) (Peprotech, catalog number: 100-18B)
20. Epidermal growth factor (EGF) (Peprotech, catalog number: AF-100-15)
21. Triiodothyronine (thyroid hormone T3) (Sigma, catalog number: T6397)
Solutions
1. PDL coating solution (see Recipes)
2. Cell detachment solution (see Recipes)
3. Dissection medium (see Recipes)
4. Papain dissociation medium (see Recipes)
5. 12% OptiprepTM medium (see Recipes)
6. OPC medium (see Recipes)
7. Post-thaw OPC medium (see Recipes)
8. OL differentiation medium (see Recipes)
Recipes
1. PDL coating solution
Make fresh on the day of use.
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Poly-D-Lysine | 0.01 mg/mL | 10 mL |
| DW (autoclaved, sterile) | n/a | 90 mL |
| Total | n/a | 100 mL |
2. Cell detachment solution
Make fresh on the day of use.
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Accumax | n/a | 10 mL |
| PBS | n/a | 20 mL |
| Total | n/a | 30 mL |
3. Dissection medium
Make fresh on the day of use. Keep in a 37 °C water bath until the moment of use.
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Penicillin-streptomycin (100×) | 1× | 1 mL |
| GlutaMAXTM (100×) | 1× | 1 mL |
| Hibernate-A medium | n/a | 98 mL |
| Total | n/a | 100 mL |
4. Papain dissociation medium
Make fresh on the day of use. Activate in a 30 °C water bath for 30 min before use. The initially opaque medium should be translucent by the time of use. While the optimal temperature for the activation and usage of papain is 30 °C, 37 °C is a usable alternative for simplicity.
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Papain suspension (~20 units/mg protein) | ~1 mg protein/mL | 100 μL |
| Dissection medium | n/a | 4 mL |
| Total | n/a | 4.1 mL |
5. 12% OptiprepTM medium
Make fresh on the day of use. Invert 3–5 times to obtain a homogenous mixture.
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| OptiprepTM | 12% | 1.2 mL |
| Dissection medium | n/a | 8.8 mL |
| Total | n/a | 10 mL |
6. OPC medium
Make fresh on the day of use. Invert 3–5 times to obtain a homogenous mixture.
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Penicillin-streptomycin (100×) | 1× | 1 mL |
| GlutaMAXTM (100×) | 1× | 1 mL |
| B27 supplement (50×) | 1× | 2 mL |
| PDGF-AA (30 μg/mL) | 30 ng/mL | 100 μL |
| bFGF (10 μg/mL) | 10 ng/mL | 100 μL |
| EGF (10 μg/mL) | 10 ng/mL | 100 μL |
| DMEM/F12 | n/a | 95.7 mL |
| Total | n/a | 100 mL |
7. Post-thaw OPC medium
Make fresh on the day of use. Invert 3–5 times to obtain a homogenous mixture.
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Penicillin-streptomycin (100×) | 1× | 1 mL |
| GlutaMAXTM (100×) | 1× | 1 mL |
| B27 supplement (50×) | 1× | 2 mL |
| PDGF-AA (30 μg/mL) | 30 ng/mL | 100 μL |
| bFGF (10 μg/mL) | 10 ng/mL | 100 μL |
| DMEM/F12 | n/a | 95.8 mL |
| Total | n/a | 100 mL |
8. OL differentiation medium
Make fresh on the day of use. Invert 3–5 times to obtain a homogenous mixture.
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Penicillin-streptomycin (100×) | 1× | 1 mL |
| GlutaMAXTM (100×) | 1× | 1 mL |
| B27 supplement (50×) | 1× | 2 mL |
| N2 supplement (100×) | 1× | 1 mL |
| Triiodothyronine (40 μg/mL) | 40 ng/mL | 100 μL |
| Neurobasal medium | n/a | 94.9 mL |
| Total | n/a | 100 mL |
Laboratory supplies
1. T75 culture flasks (vent-seal) (Corning, catalog number: 430641U)
2. Culture surfaces (plates, coverslips) (SPL Life Sciences, catalog numbers: 30012, 30024, 20009)
3. 10 cm Petri dishes (SPL Life Sciences, catalog number: 10093)
4. Conical tubes (15 and 50 mL) (SPL Life Sciences, catalog numbers: 50015, 50050)
5. Cryopreservation vials (Thermo Fisher Scientific, catalog number: 368632)
Equipment
1. Surgical scissors (large, small) (Fine Science Tools, catalog numbers: 14001-14, 14558-11)
2. Forceps (Fine Science Tools, catalog number: 11000-16)
3. Curved forceps (Fine Science Tools, catalog number: 91197-00)
4. Freezing container (Thermo Fisher Scientific, catalog number: 5100-0001)
5. Cell culture incubator (37 °C, 5% CO2) (PHCbi, catalog number: MCO-18AC-PK)
6. Water bath (Coretech, catalog number: HQ-DW22)
7. Tabletop centrifuge (compatible with 15 mL conical tubes) (Labogene, catalog number: 416)
8. LUNA-FLTM dual fluorescence cell counter (Logos Biosystems, catalog number: L20001)
9. Brightfield microscope (Olympus, catalog number: CKX53)
10. Laminar flow hood
11. -80 °C freezer
Procedure
文章信息
稿件历史记录
提交日期: Apr 4, 2025
接收日期: May 15, 2025
在线发布日期: May 30, 2025
出版日期: Jun 20, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Kim, H., Afzal, R., Kim, B. J., Cho, H. J. and Choi, J. Y. (2025). Cryopreservation of Bulk-Produced Primary Rat Oligodendrocyte Progenitor Cells. Bio-protocol 15(12): e5345. DOI: 10.21769/BioProtoc.5345.
分类
神经科学 > 细胞机理 > 细胞分离和培养
细胞生物学 > 细胞分离和培养 > 低温贮存
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link