- EN - English
- CN - 中文
Surgical Implantation of a Telemetry-Based Pressure Sensor in the Internal Jugular Vein to Monitor Respiration Wirelessly
基于无线遥测压力传感器的颈内静脉植入术用于呼吸监测
发布: 2025年06月20日第15卷第12期 DOI: 10.21769/BioProtoc.5343 浏览次数: 1493
评审: Anonymous reviewer(s)
Abstract
Active sampling, such as respiration, is known to play a major role in modulating how sensory information is perceived and encoded in the field of olfaction. Hence, monitoring respiration is crucial for understanding olfactory-guided behavior and physiology. Several methods used to measure respiration, such as infrared cameras, piezoelectric sensors, video monitoring, temperature probes, intubation, and intranasal cannula, require the animal or at least its head to be fixed. However, telemetry-based sensors can be used wirelessly, allowing animals to move freely. Here, we describe the surgical protocol to implant a telemetry pressure sensor in the internal jugular vein to detect changes in thoracic pressure. The sensor can thus help in monitoring respiration by transmitting the signal wirelessly. We describe a way of inserting the probe into the right jugular vein aseptically while housing the transmitter underneath the skin on the back of the animal. Next, based on the optimal spot for the best signal, we secure the position of the probe and suture the skin. The animal then undergoes regular post-operative care with painkillers and soft diets for up to a week. The method offers two main advantages; first, it uses a strategy similar to the jugular vein catheterization, which is widely established in rodents. Second, it minimizes the need for extensive post-operative care, including not having to shift to a liquid diet post-recovery. This makes the animals fit for most behavioral experiments requiring water or food restrictions.
Key features
• Based on Dasgupta et al. [1], this protocol describes the surgical procedure involved in the aseptic implantation of the telemetry-based pressure sensor in the internal jugular vein.
• Reduces the need for extensive post-operative care and eliminates dietary restrictions post-recovery.
• The procedure helps in obtaining mice with an implanted sensor for wireless respiration recording.
Keywords: Wireless respiration recording (无线呼吸记录)Graphical overview
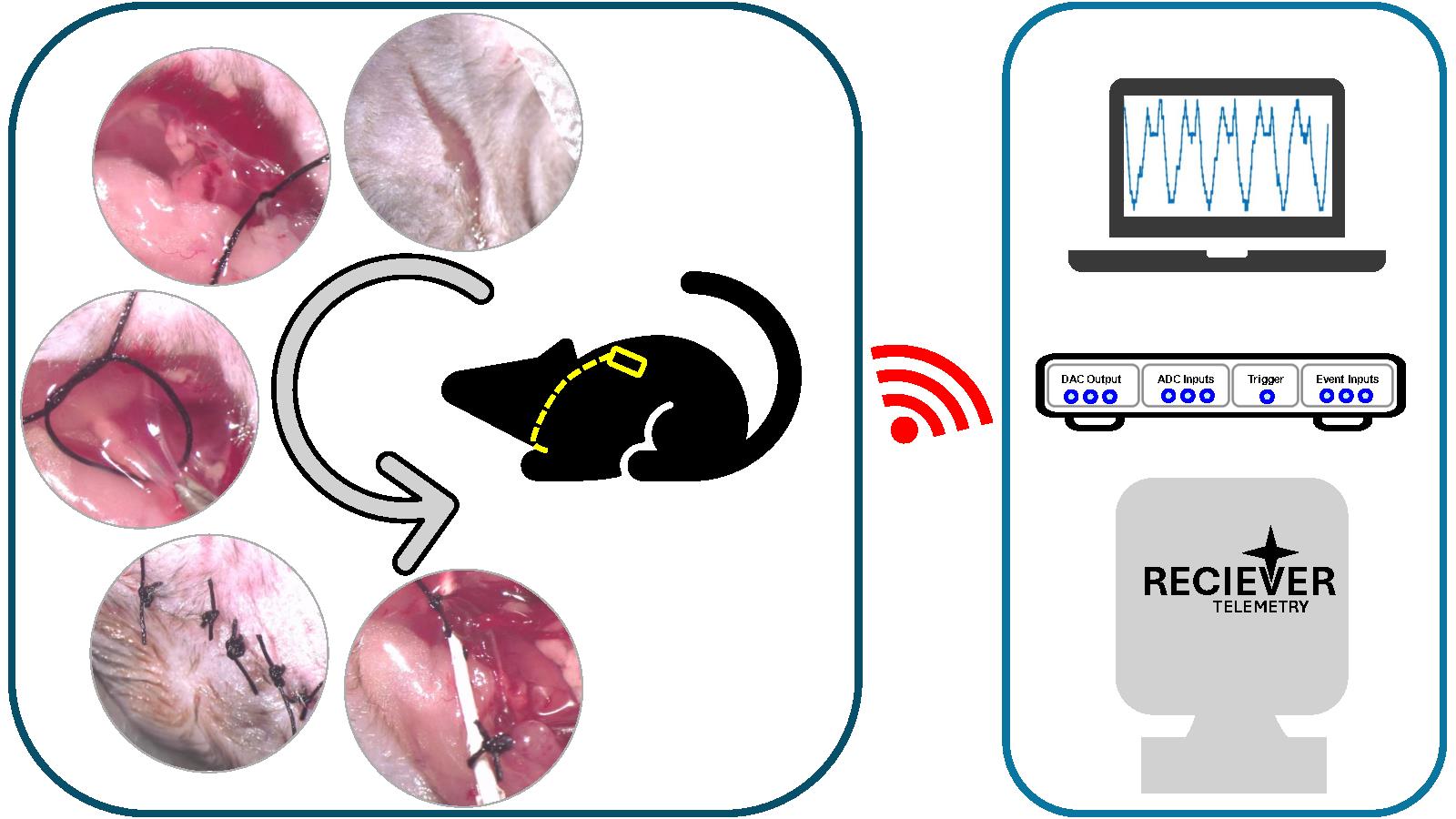
Background
Breathing patterns have been linked to rhythms occurring at different regions of the brain [2] and have been associated with distinct physiological states, e.g., sleeping [1] and attention [3], and diseased states [4], e.g., Parkinson's disease [4–6] and Alzheimer's disease [7–9]. Given that respiration has been observed to be coupled with such different behavioral states, it is important to monitor it simultaneously while estimating other behavioral and physiological parameters. Different methods have been developed to monitor respiration in different model organisms [10]. In rodents, some of those methods [11] are whole-body plethysmography [12], infrared cameras [13], piezoelectric sensors [14–16], video monitoring [17], temperature probes [18], intubation [19], intranasal cannula for flow/pressure sensing [19], face masks [20,21], and telemetry-based sensing [1,22]. However, only a few can be used for freely behaving animals, which is critical during ethologically relevant tasks like navigation.
The mammalian olfactory system has been observed to be capable of perceiving and encoding odors with a high temporal bandwidth [23–26], which gets multiplexed with the sampling rate, which is controlled by the respiration frequency [27–29]. Navigation is a critical feature in animals, and the use of the sense of smell in navigation has been observed in different organisms [30–32], including humans [33–35]. Monitoring respiration during such odor-guided tasks is critical, as active sampling has been observed to be crucial in modulating sensory perception [1,29,36–40]. These experiments would require unrestrained movement of the animal, thus requiring an untethered method of monitoring respiration. Most of the usual respiration monitoring methods require the animal to be restricted at least to the extent of head fixation and hence are not suitable for tasks involving freely moving mice.
In 2014, Reisert et al. came up with a novel method that required implanting a telemetry pressure sensor in the thoracic cavity alongside the wall of the esophagus [22]. However, this method required intensive post-operative care and switching to a liquid diet post-surgery. This can be a problem for behavioral training protocols requiring water restriction. Here, we describe a protocol that uses a novel surgical approach that reduces post-operative care and the need for a liquid diet after surgery. We established a way to implant the pressure sensor tip of the probe through the internal jugular vein into the thoracic cavity while securing the transmitter on the back of the animal underneath the skin. After recovery, the animal can lead a normal life without any diet restrictions and hence can be used for regular behavioral assays.
Materials and reagents
Biological materials
1. 6–8 weeks old mice (C57/Bl6) (males and females)
Reagents
Pre-surgery
1. Meloxicam oral suspension (1.5 mg/mL) (Metacam, Boehringer Ingelheim Animal Health USA Inc., Item code: NDC:0010-6015)
2. Meloxicam for injection (5 mg/mL) (Metacam, Boehringer Ingelheim Animal Health USA Inc., Item code: NDC:0010-6013)
3. Fentanyl (Hameln Pharma, ATC code: N01AH01)
4. Midazolam (Hameln Pharma, ATC code: N05CD08)
5. Medetomidine (Virbac, CAS No.: 86347-14-0)
6. Chlorhexidine (Merck, CAS No.: 55-56-1)
7. Chloramphenicol (eye ointment) (Ethypharm, ATC code: S01AA01)
8. Egg custard (for babies) (Cowgate)
9. Buprenorphine (Reckitt & Colman, CAS No.: 52485-79-7)
10. Trigene/Anistel (Ceva Animal Health Pty Ltd, catalog number: APVMA59998)
Surgery
1. NaCl (Merck, CAS No.: 7647-14-5)
Post-surgery
1. Naloxone (Hameln Pharma, ATC code: V03AB15)
2. Flumazenil (Hameln Pharma, ATC code: V03AB25)
3. Atipamezole (Dechra, product code: 0DEA070)
4. Emla cream (Aspen, ATC code: N01BB20)
Solutions
1. Sterile saline solution (see Recipes)
2. Anesthesia mix solution (see Recipes)
3. Wake mix solution (see Recipes)
4. Oral analgesic (see Recipes)
5. Chlorhexidine solution (see Recipes)
Recipes
1. Sterile saline solution
| Reagent | Final concentration | Quantity or volume |
| NaCl | 0.9% (w/v) | 8.5g |
| Distilled water | - | 1 L |
| Total | - | 1 L |
2. Anesthesia mix solution
| Reagent | Final concentration | Quantity or volume |
| Water for injection (sterile) | - | 15 mL |
| Fentanyl | 50 μg/mL | 2 mL |
| Midazolam | 5 mg/mL | 2 mL |
| Medetomidine | 1 mg/mL | 1 mL |
| Total | - | 20 mL |
Notes:
1. Anesthesia mix working dose is 0.05 mg/kg fentanyl + 5 mg/kg midazolam + 0.5 mg/kg medetomidine.
2. Inject 0.1 mL of anesthesia mix per 10 g of mouse body weight.
3. Wake mix solution
| Reagent | Final concentration | Quantity or volume |
| Flumazenil | 0.1 mg/mL | 10 mL |
| Naloxone | 400 μg/mL | 6 mL |
| Water for injection (sterile) | - | 3 mL |
| Atipamezole | 5 mg/mL | 1 mL |
| Total | - | 20 mL |
Notes:
1. Wake mix working dose is 1.2 mg/kg naloxone + 0.5 mg/kg flumazenil + 2.5 mg/kg atipamezole.
2. Inject 0.1 mL of wake mix per 10 g of mouse body weight.
4. Oral analgesic
| Reagent | Final concentration | Quantity or volume |
| Metacam oral suspension | 1.5 mg/mL | 1 mL |
| Egg custard | - | 1 mL |
| Total | - | 2 mL |
5. Chlorhexidine solution (25% v/v)
| Reagent | Final concentration | Quantity or volume |
| Chlorhexidine | - | 25 mL |
| Water | - | 75 mL |
| Total Volume | - | 100 mL |
Laboratory supplies
1. 500 mL plastic beaker (Merck, catalog number: BR90224)
2. 50 mL glass beaker (Merck, catalog number: CLS100050)
3. 50 mL centrifuge tubes (Merck, catalog number: CLS430829)
4. Sterile drape non-absorbent 35 × 50 cm (Vet Direct, catalog number: DRP005)
5. Syringe, 1 and 3 mL (BD microfine, catalog number: 00382903096244 and 00382903096565)
6. Transparent film (Glad Press N Seal) (The Glad Products Company, catalog number: TMMDH)
7. Suture thread (6-O silk) (Ethicon, Johnson & Johnson, USA, catalog number: NW5029)
8. Sterile cotton buds (Medline Scientific, SKU: 300230S)
9. Face mask (MediSupplies, catalog number: AEC8720)
10. Sterile gloves (MediSupplies, catalog number: PMC3066)
11. Non-sterile gloves (Merck, catalog number: Z412392)
12. Non-woven swabs (MediSupplies, catalog number: PMC1987)
Equipment
Pre-surgery
1. Mice hair shaver (Wella Professionals, model: Contura Hair Clipper)
2. Weighing scale (Merck, model: CR221)
Surgery
1. Stereomicroscope (Leica Microsystems, model: M80)
2. Micro Serrefines (venous clips) (Fine Science Tools, catalog number: 18055-01) (Figure 1A7).
3. Extra fine Graefe forceps (Fine Science Tools, catalog number: 11151-10) (Figure 1A4).
4. Adson tissue forceps, 12.0 cm, 1 × 2 teeth, with suture platform, 2 stoppings, 0.8 mm (blunt forceps) (Harvard Apparatus, catalog number: ST2 72-8573) (Figure 1A2).
5. Jeweler’s forceps, titanium, 11.0 cm, No. 4, extra delicate tips, 45°angle (Harvard Apparatus, catalog number: ST2 72-0461) (Figure 1A3).
6. Probe, double-ended (Harvard Apparatus, catalog number: ST2 72-8913) (Figure 1A8).
7. Eye scissors, 11.5 cm, straight (Harvard Apparatus, catalog number: ST2 72-8428) (Figure 1A5).
8. Olsen-Hegar needle holders with scissors and tungsten carbide jaws straight, serrated (mosquito scissor) (Harvard Apparatus, catalog number: ST2 72-88980)
9. Analogue to digital converter (Cambridge Electronic Design Limited, model: CED Micro1401 with ADC12 expansion)
10. Computer
11. Stellar implantable transmitter for mice (10× normal gain, TSE Systems, catalog number: E-430001-IMP-22) (Figure 1B).
12. Stellar receiver and antenna (TSE Systems, catalog number: 430001-REC-01)
13. Temperature monitor probe (FHC, catalog number: 40-90-5D-02)
14. Heating pad (FHC, catalog number: 40-90-2-07)
15. Mice temperature controller (FHC, catalog number: 40-90-8D-DC)

Figure 1. Dissection kit and sensor. (A) Dissection kit. 1. Dissection box. 2. Adson tissue forceps. 3. Jeweler’s forceps. 4. Extra fine Graefe forceps. 5. Eye scissors. 6. Mosquito scissors. 7. Micro Serrefines (venous clips). 8. Double-ended probe. 9. 50 mL glass beaker. (B) Steller telemetry pressure sensor.
Post-surgery
1. Recovery chamber (Tecniplast, IVC recovery rack)
Software and datasets
1. Spike2 (Cambridge Electronic Design Limited, UK, Version 7)
Procedure
文章信息
稿件历史记录
提交日期: Feb 15, 2025
接收日期: May 13, 2025
在线发布日期: Jun 5, 2025
出版日期: Jun 20, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Kushwaha, N. and Dasgupta, D. (2025). Surgical Implantation of a Telemetry-Based Pressure Sensor in the Internal Jugular Vein to Monitor Respiration Wirelessly. Bio-protocol 15(12): e5343. DOI: 10.21769/BioProtoc.5343.
分类
神经科学 > 行为神经科学 > 感觉运动反应
神经科学 > 神经解剖学和神经环路
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link