- EN - English
- CN - 中文
Quantification of Folded and Misfolded Proinsulin Forms Using Nonreducing SDS-PAGE and Proinsulin-Specific Immunoblotting
利用非还原性SDS-PAGE结合胰岛素原特异性免疫印迹定量分析折叠与错误折叠的胰岛素原形式
发布: 2025年06月05日第15卷第11期 DOI: 10.21769/BioProtoc.5337 浏览次数: 1341
评审: Hemant Kumar PrajapatiSrajan KapoorPriyanka MittalAnonymous reviewer(s)
Abstract
We have observed that some proinsulin molecules in pancreatic islets and beta cell lines have incomplete or improper intramolecular disulfide bonds. These misfolded monomers can form intermolecular disulfide-linked complexes. Accurately quantifying the fraction of proinsulin molecules contained in these complexes is challenging. By proinsulin immunoblotting after nonreducing SDS-PAGE, the signal for disulfide-linked complexes can exceed the total proinsulin signal detected after reducing SDS-PAGE (i.e., overestimating the abundance of misfolded species due to antibody affinity differences). However, after modification of the SDS-PAGE and electrotransfer protocol, we have been able to more accurately estimate the fraction of proinsulin monomers folded to the native state, as well as misfolded proinsulin monomers and disulfide-linked complexes. Our improved technique offers the ability to obtain a more precise assessment of proinsulin misfolding under different environmental conditions in beta cells and normal islets and in diabetes.
Key features
• The protocol introduces a modification of a technique that enables clear separation and accurate quantification of native and non-native proinsulin monomers and aberrant disulfide-linked oligomers.
• The protocol requires modifications to the standard SDS-PAGE and electrotransfer protocol to address quantitation inaccuracies that result from variations in antibody affinity.
• Side-by-side comparison demonstrates that the standard immunoblotting method underestimates proinsulin monomers and overestimates the abundance of proinsulin disulfide-linked complexes.
• This method is applicable to the study of recombinant proinsulin in heterologous cells, pancreatic β-cell lines, rodent or human pancreatic islets, and human iPSCs.
Keywords: Proinsulin misfolding (胰岛素原错误折叠)Graphical overview
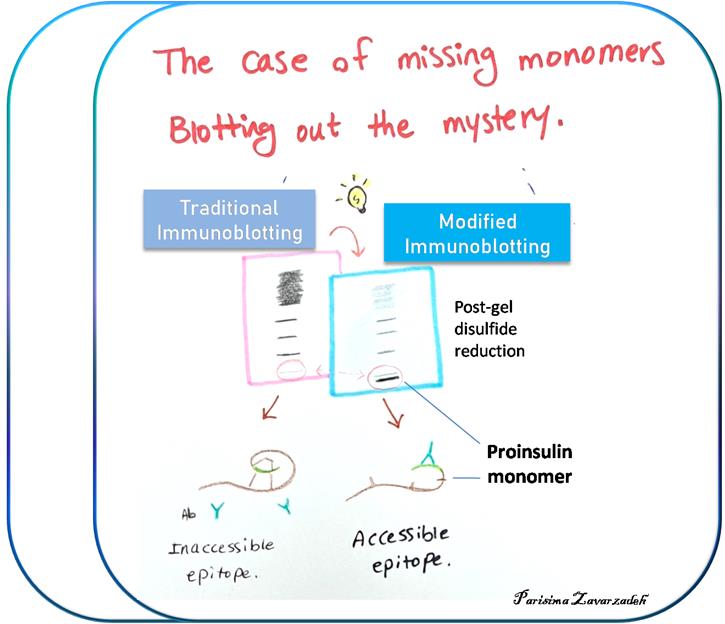
Background
In recent years, we and others have developed methods to detect non-native proinsulin in cells expressing recombinant proinsulin, pancreatic β-cell lines expressing recombinant or endogenous proinsulin, and β-cells within isolated pancreatic islets. A significant advance emerged from a simple immunoblotting technique that identified intermolecular disulfide-linked proinsulin complexes in the islets of the leptin receptor defective type-2 diabetes mouse model, db/db (LepRdb/db) [1]. When the proinsulin folding was analyzed as a function of blood glucose using this approach, it was observed that proinsulin participated in misfolding and disulfide-linked complex formation much before the onset of hyperglycemia (see Figure 1, normoglycemic db/db, glucose 101 mg/dL), indicating that proinsulin misfolding is a prelude to type-2 diabetes [1]. The immunoblotting technique to detect misfolded proinsulin in beta cells has been widely used in subsequent studies by us and others [2–6]. However, we soon realized that the original immunoblotting approach faced challenges in accurately quantifying proinsulin misfolding due to variations in the biophysical properties of different proinsulin complexes and differences in antibody affinity for these diverse folded states (Figure 1). To address these limitations, we have made key methodological refinements to enhance the accuracy in measuring the abundance of different proinsulin folded forms as separated by nonreducing SDS-PAGE. This methodology empowers any diabetes research laboratory worldwide to reliably quantify proinsulin misfolding in various contexts, including healthy and diseased human pancreas, as well as β-cell models. While techniques like differential scanning fluorimetry (DSF) can indicate changes in thermal stability potentially linked to misfolding, and circular dichroism (CD) spectroscopy can reveal alterations in secondary and tertiary structure, neither directly distinguishes between native and non-native disulfide bond arrangements or quantifies oligomeric states with the clarity offered by nonreducing SDS-PAGE. This direct visualization of disulfide-linked species makes the refined immunoblotting approach advantageous for studying the specific misfolding mechanisms of proinsulin.
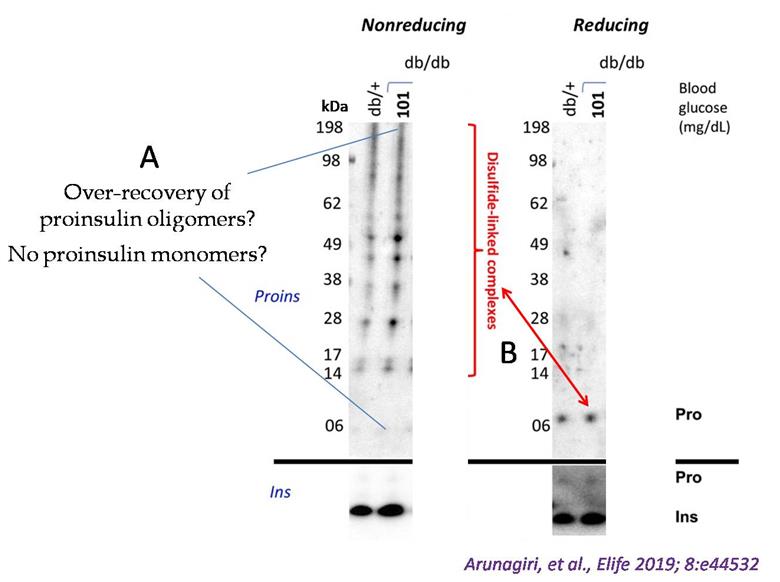
Figure 1. Detection of misfolded proinsulin in LepRdb/db mouse islets using non-reducing SDS-PAGE followed by proinsulin immunoblotting. Islet lysates from LepRdb/+ heterozygote (control, random blood glucose: 138 mg/dL) or LepRdb/db mice (random blood glucose: 101 mg/dL) were analyzed by nonreducing or reducing SDS-PAGE and immunoblotting with mAb anti-proinsulin (CCI-17, above black line; molecular mass markers are noted) or guinea pig anti-insulin (below black line). The nonreducing gel (left) exhibited two key issues. First, an apparent overrepresentation of disulfide-linked proinsulin complexes and a near absence of the monomer band. Second, the red double-headed arrow highlights a significant discrepancy: the limited total proinsulin detected under reducing conditions (right) contrasted sharply with the abundant disulfide-linked complexes observed in the nonreducing gel (left). (Modified from Arunagiri et al. [1], eLife 2019 Jun 11: 8: e44532.)
Refinement of proinsulin misfolding assay: The initial assay showed potential to identify proinsulin disulfide-linked complexes (Figure 1), but i) failed to accurately detect proinsulin monomers under nonreducing conditions, and ii) overrepresented the non-native disulfide-linked complexes, which appeared to exceed the total proinsulin recovered under reducing conditions. A revised protocol was developed to address these key limitations. A schematic comparing the traditional and the new protocol is illustrated below (Figure 2). A detailed description of the protocol is presented later in this manuscript.
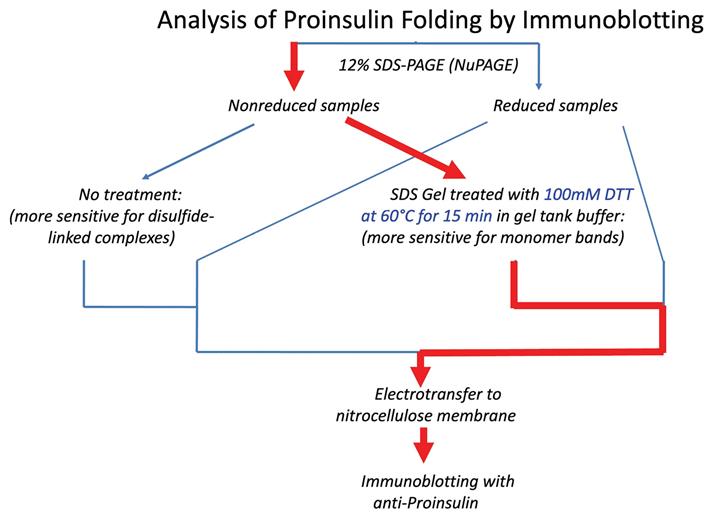
Figure 2. Schematic of different immunoblotting methods to detect proinsulin misfolding. Left: Conventional immunoblotting after nonreducing SDS-PAGE is super-sensitive to misfolded disulfide-linked complexes of proinsulin. Red arrow pathway: Treatment of samples with DTT + heating after nonreducing SDS-PAGE converts all proinsulin contained within the gel to reduced monomers. Using a fixed percentage of acrylamide ensures even transfer efficiency across the gel. This method enhances detection of proinsulin monomers (particularly native monomers) after nonreducing SDS-PAGE and provides a more quantitative estimate of the distribution of differently-folded proinsulin species. (Source: Arunagiri et al. [7], Protein Science, 33(4), e4949.)
Materials and reagents
Biological materials
1. MIN6 (mouse insulinoma) cells, obtained from Dr. D. Stoffers, U. Pennsylvania
Reagents
Chemicals
1. Dithiothreitol (DTT) (Sigma-Aldrich, catalog number: D0632); stored at 4 °C
2. Clarity Western ECL substrate (Bio-Rad, catalog number: 1705061); light sensitive, stored in the dark
3. cOmpleteTM, Mini Protease Inhibitor Cocktail (Roche, catalog number: 11836153001); stored at 4 °C
4. Bovine serum albumin (BSA), heat shock treated (Fisher BioReagentsTM, catalog number: BP1600-100); stored at 4 °C
5. Thermo ScientificTM PierceTM BCA Protein Assay kit (Fisher, catalog number: PI23227); stored at 4 °C
6. DMEM (Fisher, catalog number: 11995-065)
7. 10% fetal calf serum (BioWest, catalog number: S0400)
8. 100 IU/mL penicillin and 100 μg/mL streptomycin (Fisher, catalog number: 15140122)
9. 2-mercaptoethanol (Sigma, catalog number: M3148)
10. RIPA Buffer (Boston Bioproducts, catalog number: BP-115)
11. NuPAGETM Bis-Tris Mini Protein Gels, 12%, 1.0 mm (Fisher, catalog number: NP0342BOX)
12. NuPAGETM MES SDS running buffer (20×) (Thermo Fisher, catalog number: NP000202)
13. NuPAGETM LDS sample buffer (4×) (Thermo Fisher, catalog number: NP0007)
14. SeeBlueTM Plus2 pre-stained protein standard (Thermo Fisher, catalog number: LC5925)
15. TBST (15 mM Tris, 150 mM NaCl, 0.1% Tween-20)
Antibodies
1. Mouse mAb anti-rat proinsulin (CCI-17) (Novus Biologicals, catalog number: NB100-73013)
2. Guinea pig anti-insulin (Covance, RRID: AB_10013624)
3. Secondary antibodies:
a. Goat anti-mouse IgG-HRP Conj. (Bio-Rad, catalog number: 1706516)
b.Goat anti-guinea pig IgG (H/L), HRP (Bio-Rad, catalog number: AHP863P)
Solutions
1. Cell culture media (see Recipes)
2. DTT solution (see Recipes)
Recipes
1. Cell culture media
DMEM supplemented with 10% fetal calf serum, 100 IU/mL penicillin and 100 μg/mL streptomycin, and 0.05 mM 2-mercaptoethanol (for MIN6 cells).
2. DTT solution
Dissolve in 1× MES running buffer to a final concentration of 100 mM.
Equipment
1. XCell SureLockTM Mini-Cell mini-protein gel electrophoresis system (Thermo Fisher, model: EI0001)
2. Hoefer Semi-Dry Transfer Unit or Trans-Blot® SD Semi-Dry Transfer Cell
3. Water bath (Benchmark, model: SB0012)
4. 37 °C incubator (Thermo FormaTM, model: 3110)
5. Refrigerated centrifuge (Eppendorf, model: 5430R)
Procedure
文章信息
稿件历史记录
提交日期: Mar 16, 2025
接收日期: May 5, 2025
在线发布日期: May 26, 2025
出版日期: Jun 5, 2025
版权信息
© 2025 East Tennessee State University; This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Arunagiri, A., Jang, I., Itkin-Ansari, P., Kaufman, R. J. and Arvan, P. (2025). Quantification of Folded and Misfolded Proinsulin Forms Using Nonreducing SDS-PAGE and Proinsulin-Specific Immunoblotting. Bio-protocol 15(11): e5337. DOI: 10.21769/BioProtoc.5337.
分类
分子生物学 > 蛋白质 > 蛋白成熟
生物化学 > 蛋白质 > 结构
生物化学 > 蛋白质 > 定量
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link