- EN - English
- CN - 中文
In-house Fabrication of Nanoplastics of Tunable Composition and Application: Assessment of Bioelectric Changes in Primary Rat Lung Alveolar Epithelial Cell Monolayers Exposed to Nanoplastics
自制可调组分纳米塑料及其应用:评估其对大鼠肺泡上皮细胞单层的生物电变化影响
发布: 2025年06月05日第15卷第11期 DOI: 10.21769/BioProtoc.5329 浏览次数: 2150
评审: Chiara AmbrogioAnonymous reviewer(s)
Abstract
Plastic pollution presents a looming danger to the environment and virtually all life on planet Earth. Especially pernicious are nanoplastics (NPs), which are plastic fragments with dimensions ≤1 μm. Conventional detection methods are ineffective for NPs, while their high specific surface area renders them efficient carriers of toxic substances; additionally, they may even be inherently toxic. Although NP waste chiefly arises from environmental weathering of larger plastic fragments, most published studies employed manufactured pristine NPs of uniform size and shape. Furthermore, almost all NP effects were studied using polystyrene (PS) as a convenient model material, despite PS accounting for <6% of all plastic pollution. There is thus an urgent need to expand investigations of environmental NP pollution and effects on biota. The present work provides a comprehensive roadmap for studying the effects of “real-world” NP pollution on living systems, using, for example, lung alveolar epithelial cells on which such NPs deposit by breathing ambient air. Herein, we describe detailed in-house methods to fabricate various NPs that are weathered with UV light and O3 gas exposure to more closely mimic real environmental NPs. We also illustrate a simple and straightforward bioelectrical method for assessing passive and active ion transport properties of primary rat lung alveolar epithelial cell monolayers as a model for the distal mammalian lung exposed to one of the generated NPs. This protocol allows researchers to rapidly and more accurately assess the biological impact of various simulated environmental NPs on a vulnerable air–blood barrier in the lung.
Key features
• Many simulated weathered environmental NPs can be produced at high concentrations (up to 120 mg/mL) and yields (up to 12 mg/g bulk plastic).
• Any plastic waste can be “nano-sized” with this protocol and then studied for impacts on active and passive ion transport properties of cell monolayer models.
• Methods described herein are very relevant for studying environmental pollution effects, since NPs are found in many different shapes, sizes, and compositions.
• NP weathering and generation methods do not require any expensive or specialty lab instruments.
Keywords: In-house fabrication of weathered nanoplastics (自制风化纳米塑料)Graphical overview
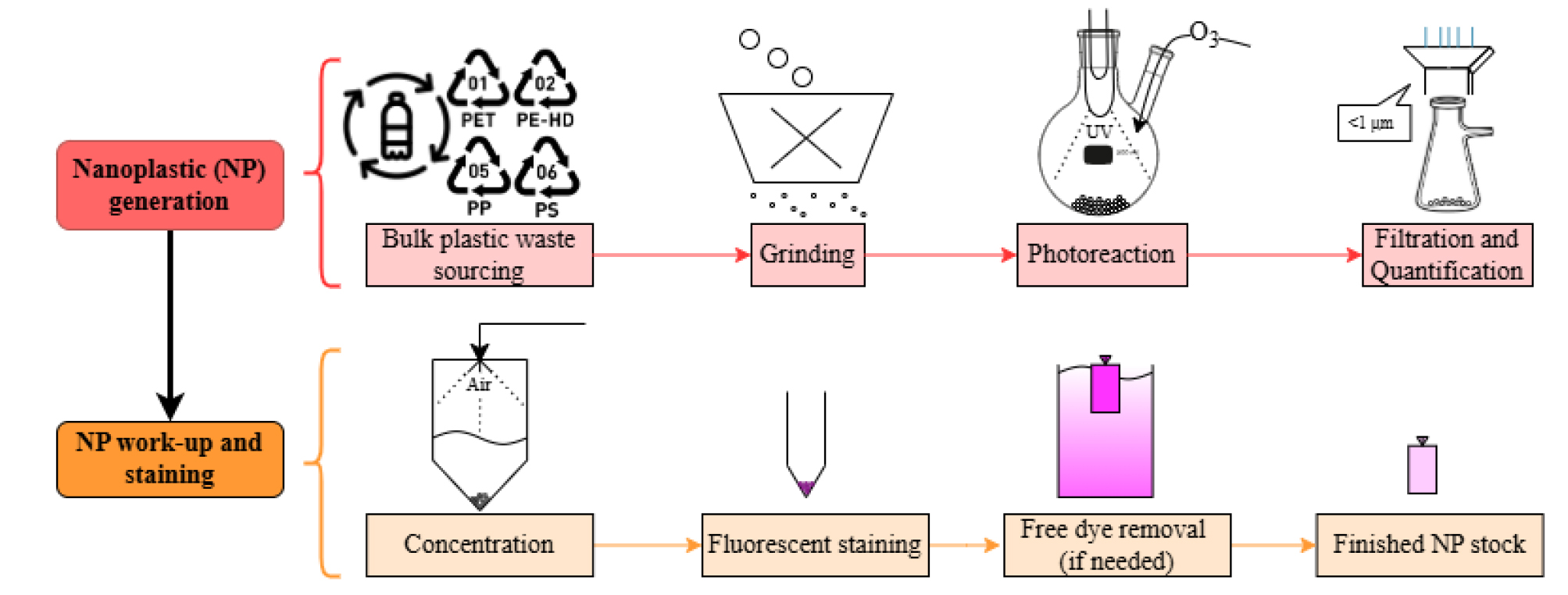
Graphical overview for in-house fabrication of nanoplastics (NP) 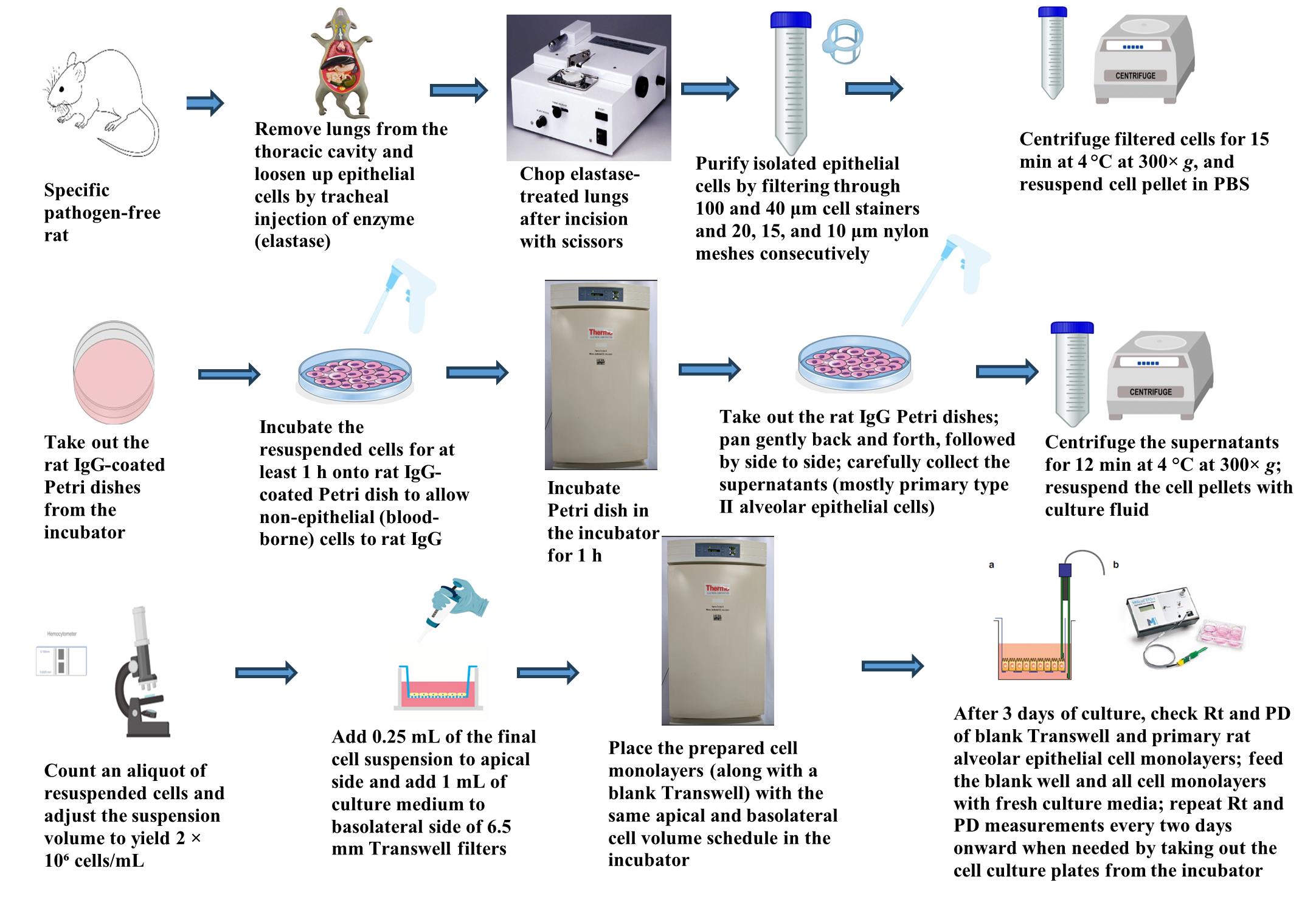
Graphical overview for primary culture of rat alveolar epithelial cells 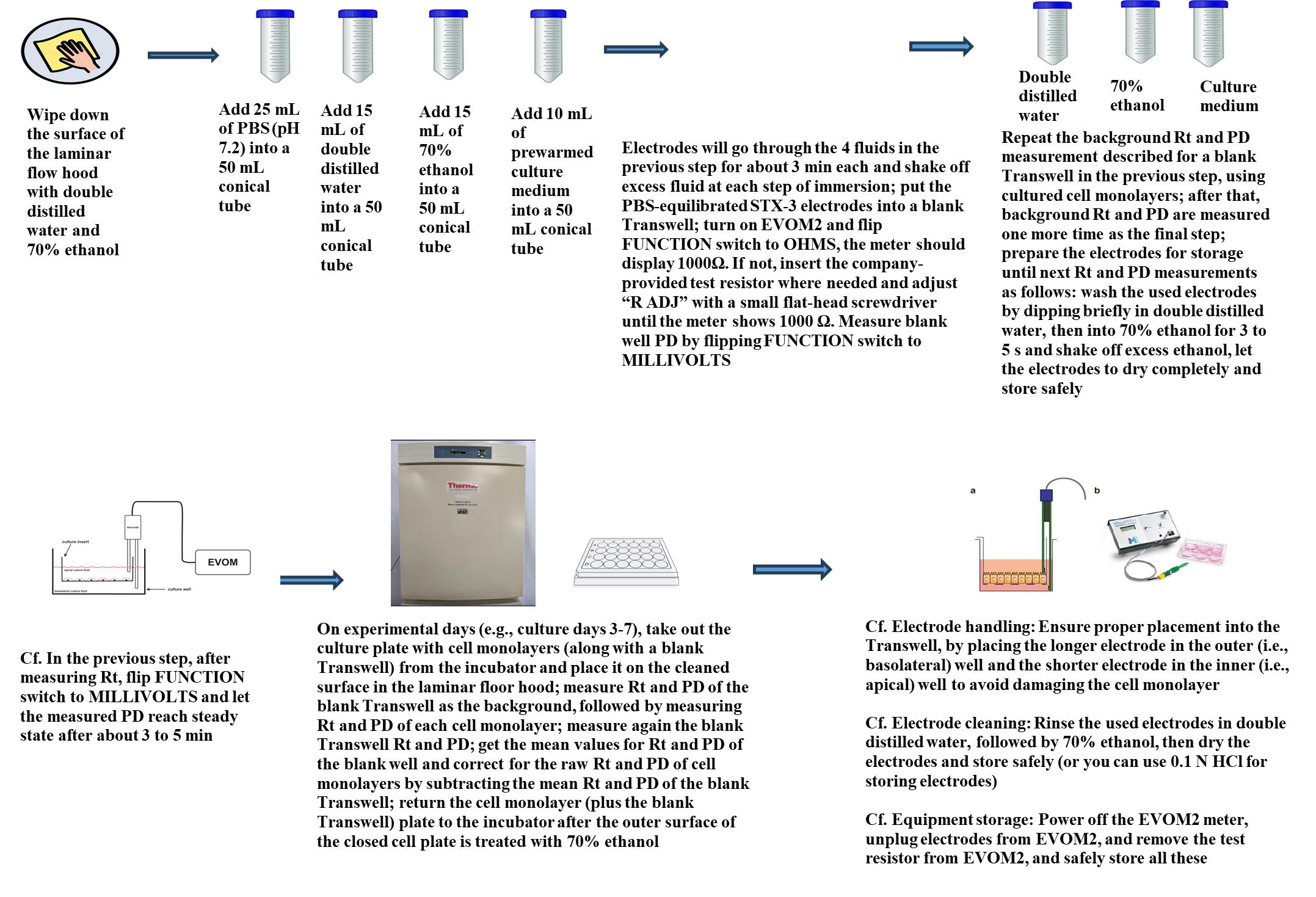
Graphical overview for measurement of Rt and PD of RAECM
Background
Waste plastic from consumer and industrial sources disperses freely into the environment, where it degrades into micro- (10–1,000 μm) and eventually nano (<1 μm) scale materials by natural weathering processes such as solar radiation and physical abrasion. These plastic fragments of micrometer and nanometer sizes are abbreviated in this article as MPs and NPs, respectively. They have become ubiquitous pollutants, with more than 250,000 metric tons of plastic estimated to persist across all waterways worldwide [1–3]. Of particular concern are NPs, because they are not only too small to be remediated by most conventional approaches [4] but are also small enough to infiltrate biological tissues and impact living systems on various cellular levels [5]. Biomimetic lipid bilayers have been extensively shown to be deformed and perforated when interacting with nanoparticles [6–11], where these damaging phenomena are reported to be driven by a combination of nanomaterial composition, size, shape, surface charge, and surface chemistry. Cell culture and toxicological studies have similarly shown various deleterious effects on biological function due to NP exposure [5,12,13].
Despite the vast differences in size, shape, and composition of environmental nanoplastic wastes, over 90% of research literature discussed in a recent review employs pristine or virgin plastic material engineered to have uniform shapes (most commonly spherical beads) and sizes. Furthermore, while polystyrene (PS) is the model plastic used in ~96% of those studies, PS comprises less than 6% of typical environmental samples [14,15]. Indeed, polystyrene (nano-PS) and polymethyl methacrylate (nano-PMMA) are often the only commercially available engineered nanoplastics [4]. The densities of nano-PS and nano-PMMA are greater than that of water/culture media, making them convenient choices for cell toxicity studies, and their particle uniformity in commercial preparations allows for predictable and consistent behavior in vivo [4]. On the other hand, “real-world” nanoplastic pollution is found with a variety of different compositions, shapes, surface chemistries, surface charges, additives, and adulterants due to the chaotic nature of environmental aging and the wide variety of plastic waste source streams [14]. Such adulterant matrices include, but are not limited to, non-plastic organic debris, including leaves and other plant matter, animal protein and remains, humic acid, soil, sediment, motor oil, bacteria, metals, paper, and fibers like cotton and rope from the environment that can be found alongside plastic waste [4]. There is thus a significant gap in understanding the biological impact of weathered waste NPs, as the vast majority of current literature uses NP models that are not environmentally realistic. In other words, those studies can demonstrate an impact on living systems, but because plastic waste is seldom uniformly shaped and pristine, further studies may be needed to determine real-world effects.
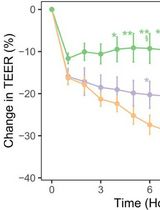
In this protocol, we describe a straightforward, customizable, and more environmentally relevant method to experimentally monitor the effects of nanoplastic pollution using, for example, rat alveolar epithelial cell monolayers for assessment of active and passive ion transport properties. NPs are generated by accelerated photoaging/photooxidation of pulverized plastic waste using an ultraviolet (UV) light source coupled with an ozone (O3) gas generator. It is estimated that this aging process simulates years of solar irradiation within an exposure time of just 72–144 h [16]. NPs generated by this method can be easily concentrated (up to ~120 mg/mL) and mixed with other NP materials as desired. Changes in cell functions (e.g., ion transport and membrane damage) of lung alveolar epithelial cell monolayers exposed apically to these different environmental NP contaminant models can then be monitored using a simple bioelectric assay by tracking both spontaneous potential difference (PD) and electrical resistance (Rt) of cell monolayers over time at different concentrations of various fabricated NPs. An equivalent short-circuit current (Ieq), which represents the active ion transport rate across the monolayer, can be estimated from the ratio PD/Rt as described previously [17,21–23]. Finally, NPs can be tagged with a fluorescent labeling molecule for convenient tracking, if needed. This will allow researchers using confocal microscopy to easily visualize NPs interacting with cells, to quantify NPs with particle counters, to track the flux of NPs across cell monolayers [17,21], and/or to further shed light on mechanisms underlying NP interactions with cells [e.g., NP interaction with lipid bilayers, endocytosis, autophagy, intracellular organelles (mitochondria, endoplasmic reticulum, Golgi, and lysosomes), and exocytosis].
The method for nanoplastic generation described herein is an improved and expanded version of the method described by Sorasan and coworkers [16]. In that original method, only PE, PP, and PS were considered, and the raw plastic materials were sourced from various plastic-strewn beaches in Spain and Portugal. While true environmental samples are the best models for studying NP pollution impact, collecting field samples can be an expensive and arduous task, since alternative methods such as Fourier transform infrared spectroscopy (FTIR) must be used to first identify the plastics found. In addition, the composition of plastics globally found in the environment is mostly PE and PP [14,16] but will vary widely among local sources. The method described herein is much more rapid, high-throughput, controllable, and cost-effective, as the researcher can “mix and match” any consumer plastic sources and have total control over the composition of the NP mixtures [e.g., PE, PP, PS, or polyethylene terephthalate (PET); any plastic amenable to grinding (e.g., PMMA, polycarbonate and nylon) can also work]. Furthermore, much less field travel time and collection and processing efforts are required. Finally, if desired, the researcher could easily adapt this procedure for field-collected samples, with the only difference being the sourcing of the plastic.
Part I: In-house fabrication and fluorescence labeling of various NPs
Materials and reagents
Reagents
1. Nile Red (Santa Cruz Biotech, catalog number: sc-203747 through sc-203747C)
2. Methanol (100%) (VWR, catalog number: BDH1135)
Solutions
1. Nile Red (1 mg/mL) in pure methanol (see Recipes)
Recipes
1. Nile Red (1 mg/mL) in pure methanol
Weigh 1.5 mg of Nile Red and place it into a glass amber vial. Add 1.5 mL of methanol (100%) into the vial and mix well to dissolve completely.
Laboratory supplies
1. Centrifuge tubes, 15 mL (VWR, catalog number: 89039-670)
2. Dye removal/size-exclusion columns, 2 mL (Thermo-Fisher, catalog number: A44299)
3. Centrifuge tubes, 50 mL (VWR, catalog number: 89079-494)
4. Microcentrifuge tubes, 1.7 mL (VWR, catalog number: 87003-294)
5. Two-necked round-bottom glass flask, 250 mL (Chemglass Life Sciences, catalog number: CG-1520-04)
6. Single-necked flat-bottom glass flask, 250 mL (Chemglass Life Sciences, catalog number: CG-1500-03)
7. Desired plastic waste for nano-sizing. Example sources include:
a. For polyethylene (PE): clear plastic transfer pipettes (Copan, catalog number: 200C or 201C)
b. For polypropylene (PP): clear microcentrifuge tubes (VWR, catalog number: 87003-294)
c. For polystyrene (PS): disposable cuvettes (Brand GmbH, catalog number: 759076D)
d. For polyethylene terephthalate (PET): clear disposable water bottles and clear food takeout containers marked with Resin Identification Code “1”
8. Polyethersulfone filters, 47 mm diameter (Pall Corporation, catalog number: 60110)
9. Dialysis cassettes, 3500 MWCO (Thermo Scientific, catalog number: PIA52966)
Equipment
1. Hg or Xe or Hg-Xe light source (Oriel Instruments, lamp numbers: 6291, 6292, 66142, 6293, 6295, 62712, or equivalent; Oriel instruments housing unit model 66902; see General notes and troubleshooting for guidelines on lamp selection) and power supply (Newport Corporation, model: 69910)
2. Ozone generator (A2Z Ozone, model: Aqua-6)
3. Ultrasonicator (Branson Ultrasonics, model: Sonifier S-450) with ~2.32 mm tip
4. Benchtop cyclone evaporator (BioChromato; model: S1-SU-120-A)
5. Vacuum filtration assembly: 1 L holding flask, 300 mL filtration funnel, 47 mm glass support base, and 47 mm aluminum clamp (Eisco Scientific, catalog number: FSAS16)
6. Block heater (VWR, catalog number: 75838-318)
7. Solid particle grinder or blender (e.g., coffee grinder, Hamilton Beach, model: 80350G)
8. Dynamic light scattering (DLS) and zeta-sizer instrument (Wyatt Technology Corporation, model: Mobius 305)
9. Other standard lab equipment [e.g., personal protective equipment (PPE), UV-resistant goggles, hotplate-magnetic stir plate, ring stand clamps, ½ inch ID (6.35 mm) vacuum hose, house vacuum line, house air line, and fume hood]
10. Centrifuge (e.g., Eppendorf, model: 5920 R)
11. Milli-Q water dispenser (e.g., Millipore, model: Advantage A10)
Software and datasets
1. DYNAMICS software (Wyatt Technology Corporation)
2. ImageJ (National Institutes of Health)
Note: With confocal laser scanning microscopy (CLSM) of Nile Red (NR)-labeled NP inside RAECM (see Figure 8), Image J can be used to quantify the fluorescence as needed (e.g., mitochondrial association of nanoplastics vs lysosome-associated nanoplastics).
3. Excel program (Microsoft)
4. Prism program version 10 (GraphPad Software)
Procedure
文章信息
稿件历史记录
提交日期: Jan 30, 2025
接收日期: Apr 26, 2025
在线发布日期: May 18, 2025
出版日期: Jun 5, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/).
如何引用
Chairil, R., Alvarez, J. R., Sipos, A., Malmstadt, N., Crandall, E. D. and Kim, K. J. (2025). In-house Fabrication of Nanoplastics of Tunable Composition and Application: Assessment of Bioelectric Changes in Primary Rat Lung Alveolar Epithelial Cell Monolayers Exposed to Nanoplastics. Bio-protocol 15(11): e5329. DOI: 10.21769/BioProtoc.5329.
分类
环境生物学 > 生态系统
细胞生物学 > 基于细胞的分析方法 > 电生理技术
生物工程
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link