- EN - English
- CN - 中文
Electrophysiological Evaluation of a Sciatic Nerve Degree III Injury Model in Rats
大鼠坐骨神经III级损伤模型的电生理评估
发布: 2025年05月20日第15卷第10期 DOI: 10.21769/BioProtoc.5311 浏览次数: 1794
评审: Olga KopachAnonymous reviewer(s)
Abstract
Sciatic nerve injury is a prevalent traumatic condition that significantly impacts a patient's quality of life. The sciatic nerve compression injury model is among the most commonly utilized models for investigating nerve repair and regeneration. Within this context, the degree III sciatic nerve injury model is frequently employed in scientific research due to its clinical relevance and its suitability for studies focused on functional recovery. However, a standardized approach for accurately assessing the success of constructing the degree III sciatic nerve injury model remains lacking. Traditional macroscopic observation methods exhibit limitations, whereas neurophysiological testing serves as a highly sensitive and objective evaluation technique that can directly reflect changes in nerve conduction function, thus providing reliable quantitative evidence for the successful establishment of the model. This study aims to offer a comprehensive description of the application of neurophysiological techniques in evaluating the construction of the degree III sciatic nerve injury model, thereby ensuring the success of model preparation.
Key features
• Precisely simulate a degree III sciatic nerve injury.
• Conduct neurophysiological tests on the sciatic nerve 30 min after the injury.
• Use neurophysiological techniques to improve the accuracy and repeatability of scientific study results.
Keywords: Sciatic nerve (坐骨神经)Graphical overview
Background
In clinical practice, sciatic nerve injury is a prevalent traumatic disorder frequently resulting from sports injuries, industrial accidents, or traffic incidents [1]. These injuries can lead to impairments in both motor and sensory functions in the regions innervated by the affected nerve [2]. Consequently, the field of neuroscience research has focused on the rehabilitation of sciatic nerve injuries.
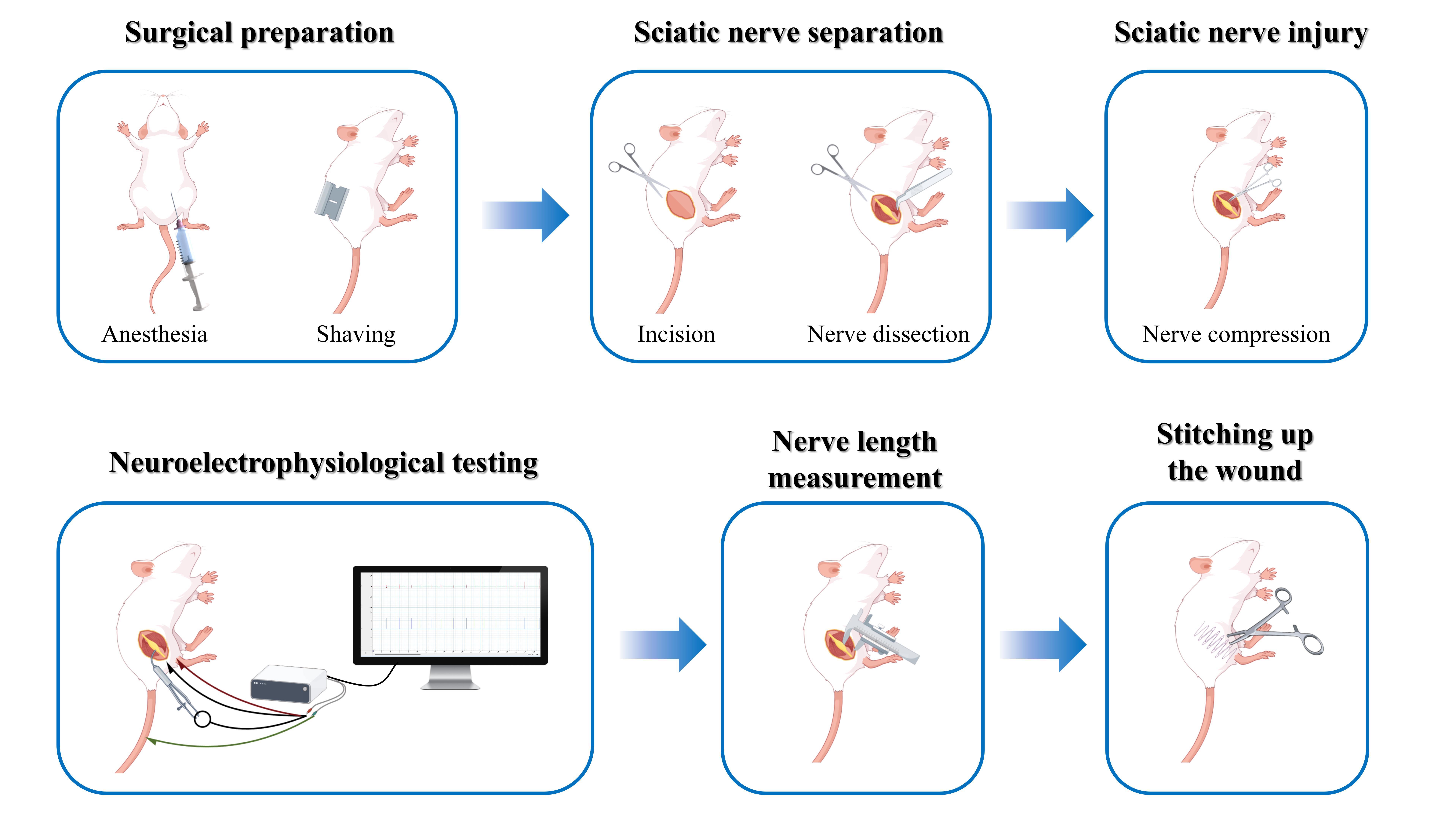
Investigating nerve repair and regeneration in vitro poses significant challenges, which often makes animal experiments the preferred research method. The rat sciatic nerve injury model is particularly favored due to its ease of operation and its similarity to human nerve distribution [3]. According to Sunderland's degree V nerve injury classification [4], a degree III injury involves a rupture of both the nerve axon and the intima, resulting in structural disarray and incomplete spontaneous recovery. This type of injury is commonly encountered in clinical practice, particularly those resulting from trauma [5]. In models of degree III nerve injury, hemostatic forceps are frequently utilized to induce the injury. This technique is straightforward, allowing for the preservation of the epineurium’s integrity by carefully controlling the force and duration of the clamp, thus maintaining a degree of connectivity between the proximal and distal ends of the injury site [6]. However, following compression with a hemostat, some nerve fibers may only temporarily lose their conductive function, and the subsequent recovery of nerve function may not necessarily be attributable to the regeneration of severed axons [7]. Consequently, accurately assessing the successful construction of the sciatic nerve degree III injury model remains a challenging task.
The traditional assessment method for nerve damage relies heavily on visual observation, which complicates the accurate evaluation of the true condition of nerve injury. Existing literature indicates that neuroelectrophysiological testing is an effective method for assessing the functional status of nerves by recording nerve conduction velocity and action potential amplitude. This approach directly reflects changes in nerve conduction function and offers significant advantages in determining the extent of sciatic nerve injury [8,9]. Unlike previous methods that rely exclusively on visual inspection, this methodology is more sensitive and objective, providing credible quantitative evidence for effective model creation. A classical approach involves measuring nerve conductance by placing both recording and stimulating electrodes directly on the nerve. However, an alternative method, which involves stimulating the sciatic nerve while recording from the associated muscles (e.g., the gastrocnemius muscle), has been increasingly adopted in recent studies due to its ability to assess both nerve and muscle function simultaneously. This approach provides a more comprehensive evaluation of nerve injury and recovery, particularly in models where motor function restoration is a key outcome measure. In a study performing electrophysiological testing at 30 minutes post-induction, a reduction in nerve conduction velocity below 10 m/s demonstrated axonal disruption and endoneurial damage with preserved perineurial integrity, consistent with a Sunderland degree III sciatic nerve injury model [10]. The study established several sciatic nerve injury models based on the Sunderland degree V classification, revealing a strong correlation between nerve conduction velocity and lesion severity. Morphological findings indicated a Sunderland degree III injury characterized by nerve conduction velocity of less than 10 m/s and considerable fiber disruption, while the perineurium remained intact [11]. Consequently, the successful creation of a degree III sciatic nerve injury model can be verified through nerve electrophysiological testing of conduction velocity. However, the procedure for creating and assessing a degree III sciatic nerve injury model has not yet been detailed in a study protocol. To ensure the consistency and reproducibility of the degree III sciatic nerve damage model, this study aims to provide a comprehensive explanation of the procedure for creating and evaluating the model using nerve electrophysiology.
Materials and reagents
Biological materials
1. Male specific pathogen-free (SPF) Sprague-Dawley rats (8–9 weeks, 250–300 g) (Beijing HFK Bioscience Co., Ltd.)
Reagents
1. Physiological saline (Invitrogen, catalog number: G4702), storage temperature: 4 °C
2. Povidone-iodine disinfectant solution (Lircon, catalog number: 20020059), storage temperature: room temperature (RT)
3. 75% ethanol (BOXCON, catalog number: 75DSJJ2500ML), storage temperature: 20–25 °C
4. 1% hydrochloric acid alcohol (Byxbio, catalog number: BYX1211H), storage temperature: RT
5. 10% neutral buffered formalin (Wexis, catalog number: 21062803), storage temperature: 0–40 °C
6. 0.5% immunofluorescence permeabilization buffer (TPBS) (ReportBio, catalog number: RS0050), storage temperature: RT
7. Xylene (Sigma-Aldrich, catalog number: 247642), storage temperature: 16–26 °C
8. Absolute ethanol (Sigma-Aldrich, catalog number: E111963), storage temperature: 20–25 °C
9. Hematoxylin stain (Baso, catalog number: C240907), storage temperature: RT
10. Eosin stain (Baso, catalog number: C220905), storage temperature: 15–25 °C
11. 1× phosphate buffered saline (PBS) (Servicebio, catalog number: G4207), storage temperature: 4 °C
12. Trypsin digestion solution (Solarbio, catalog number: X1020), storage temperature: -20 °C
13. Antibody diluent (ZSGB-BIO, catalog number: ZLI-9030), storage temperature: 4 °C
14. DAPI fluoromount-G (SouthernBiotech, catalog number: 0100-20), storage temperature: 4 °C
15. Anti-S100β antibody (HUABIO, catalog number: ET1610-3), dilution ratio: 1:800, storage temperature: 4 °C
16. NF200 monoclonal antibody (Proteintech, catalog number: 60331-1-Ig), dilution ratio: 1:800, storage temperature: 4 °C
17. Donkey anti-mouse IgG (H+L) highly cross-adsorbed secondary antibody (Invitrogen, catalog number: A-21202), dilution ratio: 1:400, storage temperature: 4 °C
18. Donkey anti-rabbit IgG (H+L) highly cross-adsorbed secondary antibody (Invitrogen, catalog number: A-21207), dilution ratio: 1:400, storage temperature: 4 °C
Solutions
1. 3% sodium pentobarbital solution (see Recipes)
2. 70% ethanol (see Recipes)
3. 80% ethanol (see Recipes)
4. 90% ethanol (see Recipes)
Recipes
1. 3% sodium pentobarbital solution
Store at 4 °C in the dark for up to 1 month. Maintain sterile technique throughout the preparation process. Use containers with tight seals for storage.
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Pentobarbital sodium powder | 0.03 g/mL | 1.5 g |
| Ultrapure water | 50 mL | 50 mL |
2. 70% ethanol
Store 70% ethanol at RT (20–25 °C) in a tightly sealed container for up to 6 months. Protect it from direct sunlight and open flames due to flammability. Clearly label the container with the preparation date and concentration. Use sterile technique during handling to prevent contamination. Check for evaporation or microbial growth before use.
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Absolute ethanol | 70% | 560 mL |
| Ultrapure water | 30% | 240 mL |
3. 80% ethanol
Follow the same storage guidelines as 70% ethanol.
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Absolute ethanol | 80% | 640 mL |
| Ultrapure water | 20% | 160 mL |
4. 90% ethanol
Follow the same storage guidelines as 70% ethanol.
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Absolute ethanol | 90% | 720 mL |
| Ultrapure water | 10% | 80 mL |
Laboratory supplies
1. Sterile surgical drapes (Fisherbrand, catalog number: 22-362-178)
2. Sterile gloves (Medicom, catalog number: 201898)
3. 5 mL syringe (CHEMIL s.r.l., Padova, catalog number: S01G25)
4. 50 mL conical tubes (Biosharp, catalog number: BS-500-M)
5. Double-edged blade (Baili, catalog number: BD007C)
6. Suture needles (Binxiong, catalog number: 422105262)
7. Medical ruler (XG, catalog number: 8895)
8. 8–0 non-absorbable surgical suture (Ethicon, catalog number: VCP774D)
9. 3–0 non-absorbable surgical suture (Zhenbang, catalog number: 17Z1001J)
10. Medical cotton swabs (Hongsheng, catalog number: HS637)
11. Restraint board (Haopai, catalog number: ZK-MZJP-B)
12. 1.5 mL clear microcentrifuge tubes (Corning, catalog number: MCT-150-C)
13. Embedding cassettes (CITOTEST, catalog number: 31050102W)
14. High-profile microtome blades (Leica, catalog number: 14035838383)
15. Adhesion microscope slides (CITOTEST, catalog number: 188105W)
16. Slide staining rack (Tianzixing, catalog number: RSJ-26)
17. Transfer pipette (BKMAMLAB, catalog number: 110205007)
18. Laboratory timer (Lissa, catalog number: LSK1010)
19. Microscope cover glass (CITOTEST, catalog number: 10212450C)
20. Paraffin (Leica, catalog number: 39601006)
21. Neutral balsam (Biosharp, catalog number: BL704A)
Equipment
1. Ophthalmic scissors (Jinzhong, model: Y00030)
2. Straight forceps (Jinzhong, model: JD1050)
3. Curved forceps (Jinzhong, model: JD1060)
4. Straight sharp scissors (Jinzhong, model: J21070)
5. Needle holder (Jinzhong, model: DS5002)
6. Curved hemostat (Jinzhong, model: DS4004)
7. 14 cm hemostat clamp (Jinzhong, model: J31050)
8. Electronic balance (Leqi, model: LQ-C5001)
9. Heating pad (Jiacai, model: JRD240727)
10. Refrigerator (2–8 °C)
11. Electromyography signal acquisition system (AD Instruments, model: PL3508/P)
12. Automatic tissue processor (Leica, model: ASP200S)
13. Constant temperature incubator (Lichen, model: DZF-6210AB)
14. Orbital shaker (JOANLAB, model: OS-20)
15. Tissue embedding machine (Leica, model: EG1150)
16. Microtome (Craftek, model: CR-601ST)
17. Biological microscope (Olympus, model: DP80)
18. Rotating disc laser confocal microscope (ANDOR, model: Dragonfly)
Software and datasets
1. LabChart (version 8.1.28 Chinese, 22/12/2023, https://www.adinstruments.com)
Procedure
文章信息
稿件历史记录
提交日期: Feb 18, 2025
接收日期: Apr 11, 2025
在线发布日期: May 11, 2025
出版日期: May 20, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Chen, L., Wang, Z., Wang, C., Ma, N., Zhao, W., Liu, S., Lu, J., Zhao, B., Sun, H., Che, P. and Dou, N. (2025). Electrophysiological Evaluation of a Sciatic Nerve Degree III Injury Model in Rats. Bio-protocol 15(10): e5311. DOI: 10.21769/BioProtoc.5311.
分类
神经科学 > 周围神经系统 > 坐骨神经
分子生物学 > 蛋白质 > 表达
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link