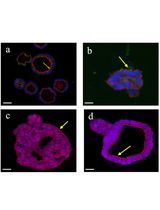
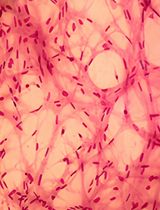
- EN - English
- CN - 中文
A Cartilaginous Organoid System Derived From Human Expanded Pluripotent Stem Cells (hEPSCs)
基于人扩增型多能干细胞(hEPSCs)构建的软骨类器官体系
(*contributed equally to this work) 发布: 2025年05月05日第15卷第9期 DOI: 10.21769/BioProtoc.5304 浏览次数: 2228
评审: Xiaokang WuKrishna Murthy NakuluriWilliam C. W. ChenPhilipp Wörsdörfer
Abstract
The development of human organotypic models of cartilage provides essential insights into chondrogenesis and chondrocyte hypertrophy while enabling advanced applications in drug discovery, gene editing, and tissue regeneration. Here, we present a robust and efficient protocol for differentiating human expanded pluripotent stem cells (hEPSCs) into hypertrophic chondrocytes through a sclerotome intermediate. The protocol involves initial sclerotome induction, followed by 3D chondrogenic culture and subsequent hypertrophic maturation induced by bone morphogenetic protein-4 (BMP4), thyroid hormone (T3), and β-glycerophosphate. This protocol also allows for sensitive testing of the effects of various compounds on hypertrophic differentiation during the maturation process. Furthermore, we identify an α-adrenergic receptor antagonist, phentolamine, as an inhibitor of hypertrophic differentiation. This organoid system provides a practical platform for exploring cartilage hypertrophy mechanisms and testing therapeutic strategies for cartilage regeneration.
Key features
• This differentiation protocol generates hypertrophic chondrocytes from hEPSCs through a sclerotome intermediate.
• This protocol facilitates sensitive testing of compounds during the hypertrophic maturation stage, enabling the study of molecular mechanisms and therapeutic interventions for cartilage hypertrophy.
• This protocol identifies the α-adrenergic receptor antagonist phentolamine as a modulator of hypertrophic differentiation.
Keywords: Human expanded pluripotent stem cells (人扩增型多能干细胞)Graphical overview
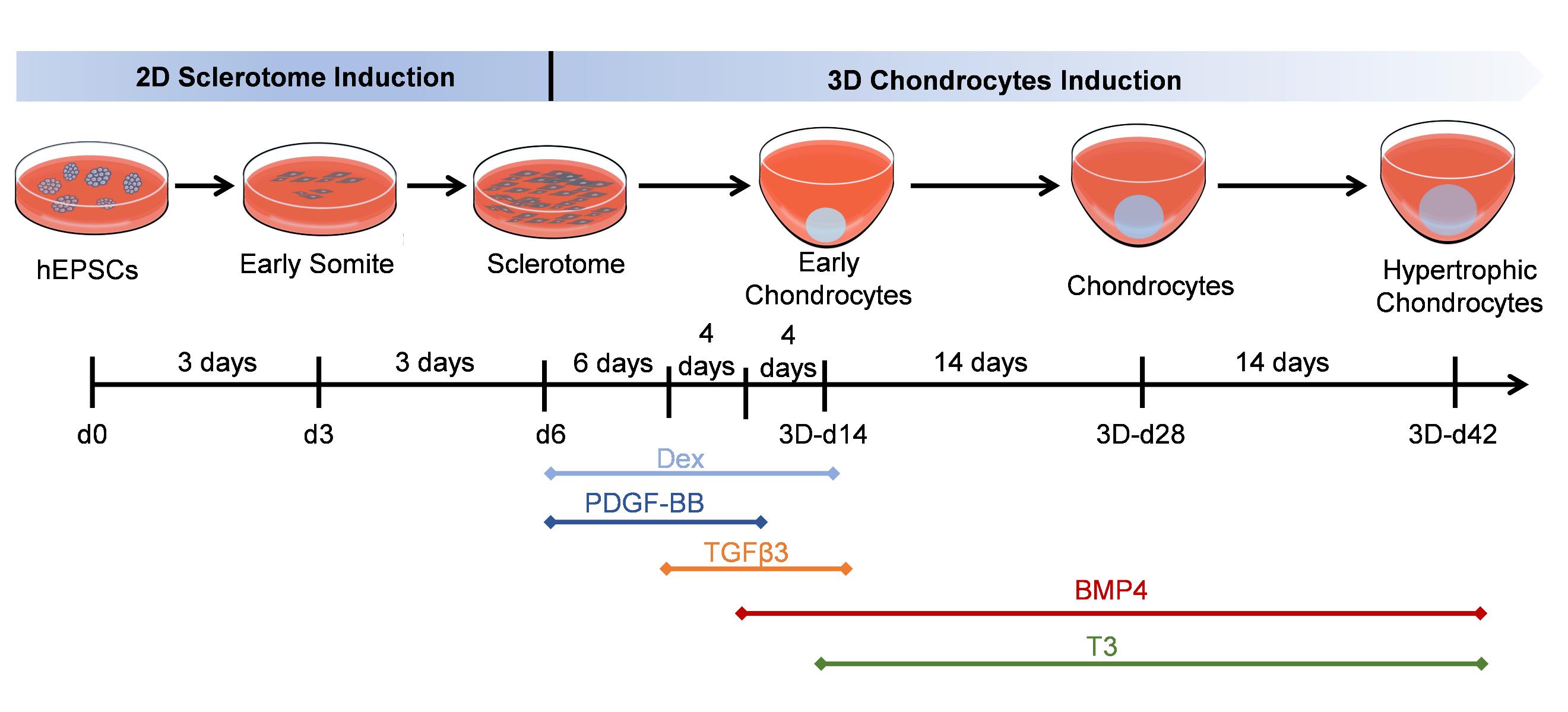
Generate hypertrophic chondrocytes from human expanded pluripotent stem cells (hEPSCs) through a sclerotome intermediate.
Background
Cartilage repair and regeneration remain significant challenges in regenerative medicine due to the limited regenerative capacity of cartilage tissues [1] and the complex biology underlying chondrogenesis and chondrocyte hypertrophy [2,3]. Human organotypic models derived from human pluripotent stem cells (hPSCs) offer a promising approach for advancing cartilage research [4,5]. Several protocols for generating hPSC-derived chondrocytes exist, including differentiation protocols based on mesodermal or sclerotomal induction [6,7], but these protocols often lack the complexity of 3D architecture and do not fully recapitulate the hypertrophic maturation stage.
Here, we provide a reproducible, efficient method for differentiating human expanded pluripotent stem cells (hEPSCs) into hypertrophic chondrocytes. EPSCs have enriched molecular signatures of blastomeres and possess developmental potency for all embryonic and extra-embryonic cell lineages. Our protocol is based on a 6-day-long sclerotome induction [8] followed by a 6+ week 3D chondrogenic culture [9]. Hypertrophic maturation is based on stage-specific administration of transforming growth factors (TGFs) and bone morphogenetic proteins (BMPs), controlling the induction of the sclerotome cells into chondrocyte differentiation and subsequent hypertrophy. After four weeks of maturation, the majority of hEPSC-derived chondrocytes express the typical chondrocyte marker type II collagen (COL2A1). Furthermore, we identify the α-adrenergic receptor antagonist phentolamine as an inhibitor of the hypertrophic chondrocyte marker type X collagen (COL10A1), demonstrating its potency in suppressing hypertrophic differentiation. This protocol systematically replicates the progression of cartilage development, from mesodermal commitment to sclerotome specification, chondroprogenitor differentiation, extracellular matrix maturation, and terminal hypertrophy, faithfully recapitulating the hierarchical stages of chondrogenesis. Additionally, this protocol enables the testing of various compounds for their effects on hypertrophic differentiation, providing a platform for drug discovery and therapeutic development.
Materials and reagents
Biological materials
1. M1 human expanded pluripotent stem cells (hEPSCs) carrying a COL2A1mCherry and COL10A1eGFP double reporter (generated in-house [10])
Reagents
1. Gelatin (Macklin, catalog number: G821281-250g)
2. Phosphate buffer saline (PBS) (Thermo Fisher Scientific, Gibco, catalog number: C10010500BT)
3. DMEM, high glucose, pyruvate (Thermo Fisher Scientific, Gibco, catalog number: C11995500BT)
4. Fetal bovine serum, qualified, Australia (FBS) (Thermo Fisher Scientific, Gibco, catalog number: 10099141C)
5. Penicillin-streptomycin (pen/strep) (5,000 U/mL) (Thermo Fisher Scientific, Gibco, catalog number: 15070063)
6. Dimethyl sulfoxide (DMSO) (Sigma-Aldrich, catalog number: D2650)
7. DMEM/F12 (Thermo Fisher Scientific, Gibco, catalog number: 21331020)
8. N-2 Supplement (100×) (N2) (Thermo Fisher Scientific, Gibco, catalog number: 17502048)
9. B-27 serum-free supplement (50×) (B27) (Thermo Fisher Scientific, Gibco, catalog number: 17504044)
10. GlutaMAX (Thermo Fisher Scientific, Gibco, catalog number: 35050061)
11. MEM non-essential amino acids solution (NEAA) (100×) (Thermo Fisher Scientific, Gibco, catalog number: 11140050)
12. L-ascorbic acid (Sigma-Aldrich, catalog number: 49752-10G, product format: 100 mg/mL in PBS)
13. XAV939 (Sigma-Aldrich, catalog number: X3004, product format: 50 mM in DMSO)
14. CHIR99021 (TOCRIS, catalog number: 4423, product format: 10 mM in DMSO)
15. A419259 (TOCRIS, catalog number 3914, product format: 5 mM in sterile H2O)
16. Human leukemia inhibitory factor (LIF) (Sigma-Aldrich, catalog number: LIF1050)
17. 2-mercaptoethanol (Sigma-Aldrich, catalog number: M6250)
18. Y-27632 (APExBIO, catalog number: A3008, 100 mM in PBS)
19. 0.25% trypsin/EDTA (Thermo Fisher Scientific, Gibco, catalog number: 25200072)
20. Matrigel (Corning, catalog number: 356230)
21. IMDM, GlutaMAXTM supplement (IMDM+Glu) (Thermo Fisher Scientific, Gibco, catalog number: 31980030)
22. Ham’s F12, GlutaMAXTM supplement (Ham’s F12+Glu) (Thermo Fisher Scientific, Gibco, catalog number: 31765035)
23. 7.5% BSA solution (Solarbio, catalog number: H1130)
24. Chemically defined lipid concentrate (CD lipid) (Thermo Fisher Scientific, Gibco, catalog number: 11905031)
25. Insulin, transferrin, selenium, ethanolamine solution (ITS-X) (Thermo Fisher Scientific, Gibco, catalog number: 51500056)
26. Monothioglycerol (Sigma-Aldrich, catalog number: M6145)
27. Activin A (R&D Systems, catalog number: 338-AC-050, product format: 100 μg/mL in sterile 4 mM HCl)
28. Recombinant human FGF basic (FGF2) (R&D Systems, catalog number: 233-FB-025, product format: 250 μg/mL in PBS containing 0.1% BSA)
29. A8301 (Sigma-Aldrich, catalog number: SML0788, product format: 10 mM in DMSO)
30. LDN193189 (Stemgent, catalog number: 04-0074, product format: 10 mM in DMSO)
31. C59 (Cellagentech, catalog number: C7641-2s, product format: 10 mM in DMSO)
32. PD173074 (TOCRIS, catalog number: 3044, product format: 10 mM in DMSO)
33. SAG (Sigma-Aldrich, catalog number: SML1314-1MG, product format: 1 mM in H2O)
34. DMEM/F-12, GlutaMAXTM supplement (DMEM/F12+Glu) (Thermo Fisher Scientific, Gibco, catalog number: 10565018)
35. Proline (Sigma-Aldrich, catalog number: P5607, product format: 50 mg/mL in PBS)
36. D -(+)-Glucose solution (glucose) (Sigma-Aldrich, catalog number: G8769, product format: 45% in H2O)
37. Sodium pyruvate (Sigma-Aldrich, catalog number: S8636)
38. Dexamethasone (Dex) (Wako, catalog number: 047-18863, product format: 1 mM in ethanol)
39. Human PDGF-BB proteins (PDGF-BB) (R&D Systems, catalog number: 220-BB-010, product format: 100 μg/mL in sterile 4 mM HCl)
40. Recombinant human TGF-beta 3 protein (TGFβ3) (R&D Systems, catalog number: 243-B3-002, product format: 20 μg/mL in sterile 4 mM HCl containing 0.1% BSA)
41. Recombinant human BMP4 protein (BMP4) (R&D Systems, catalog number: 314-BP-010, product format: 100 μg/mL in sterile 4 mM HCl containing 0.1% BSA)
42. L-3,3,5-Triiodothyronine, free acid (T3) (Sigma-Aldrich, catalog number: 642511, product format: 0.1 mM in sterile 0.1% NaOH)
43. β-glycerophosphate (Sigma-Aldrich, catalog number: 50020, product format: 1 M in DMEM/F12+Glu)
44. Phentolamine (TargetMol, catalog number: T1275, product format: 10 mM in DMSO)
Solutions
1. Gelatin coating (see Recipes)
2. Feeder medium (M10) (see Recipes)
3. hEPSC medium (hEPSCM) (see Recipes)
4. hEPSC subculture medium (see Recipes)
5. Matrigel coating (see Recipes)
6. Chemically-defined basal medium (CDBM) (see Recipes)
7. Anterior primitive streak induction medium (APSIM) (see Recipes)
8. Paraxial mesoderm induction medium (PMIM): 2D-d1 (see Recipes)
9. Early somite induction medium (ESIM): 2D-d2 (see Recipes)
10. Sclerotome induction medium (SCLIM): 2D-d3–d6 (see Recipes)
11. Chondrogenic basal medium (CBM) (see Recipes)
12. Early chondrocyte induction medium (ECIM): 3D-d0–d6 (see Recipes)
13. Chondrocyte differentiation medium 1 (CDIM1): 3D-d6–d10 (see Recipes)
14. Chondrocyte differentiation medium 2 (CDIM2): 3D-d10–d14 (see Recipes)
15. Chondrocyte maturation medium (CMM): 3D-d14–d28 (see Recipes)
16. Chondrocyte hypertrophy medium (CHM): 3D-d28–d42 (see Recipes)
Recipes
1. Gelatin coating
First, prepare a stock concentration (20×): Dilute 2 g of gelatin granules in 100 mL of H2O and autoclave. Aliquot the 20×solution in 10 mL/aliquot. Next, prepare the working concentration (1×): Dilute 2 mL of the stock concentration (20×) with 38 mL of sterile H2O to make a 1× solution. Both 20× and 1× aliquots can be stored at 4 °C.
| Reagent | Stock concentration | Quantity or Volume |
|---|---|---|
| H2O | 100% | 100 mL |
| Gel (granules) | 2% | 2 g |
| Reagent | Working concentration | Quantity or Volume |
|---|---|---|
| H2O | 99.9% | 38 mL |
| Gel (20×) | 0.1% | 2 mL |
2. Feeder medium (M10)
Prepare ~500 mL of bulk solution of DMEM-HG, FBS, and pen/strep and filter sterilize. Thaw the frozen FBS at 2–8 °C overnight. Mix the thawed FBS and pen/strep by gently inverting the vial a couple of times and then aseptically transfer it to the bottle of DMEM-HG. Swirl the bottle to mix. M10 can be stored at 2–8 °C for up to two weeks.
| Reagent | Final concentration | Volume |
|---|---|---|
| DMEM-HG | 89% | 445 mL |
| FBS | 10% | 50 mL |
| Pen/strep | 1% | 5 mL |
| Total | 100% |
3. hEPSC medium (hEPSCM)
Prepare ~500 mL of the bulk basic medium (DMEM/F12, N2, B27, GlutaMAX, pen/strep, NEAA, L-ascorbic acid) and filter sterilize. Dissolve the small molecule compounds in advance and dispense the required amounts needed to prepare 500 mL of the medium. Add fresh small molecule compounds each time the medium is prepared, avoiding repeated freezing and thawing. Combine all required ingredients into the base medium and swirl gently to mix. Dispense the medium promptly and store any unused portion at -20 °C. If needed, thaw the unused medium overnight at 2–8 °C. hEPSCM can be stored at 2–8 °C for two weeks. Filter sterilize and bring hEPSCM to room temperature before use.
| Reagent | Final concentration | Volume (for 500 mL) |
|---|---|---|
| DMEM/F12 | 95.5% | 477.5 mL |
| N2 | 0.5% | 2.5 mL |
| B27 | 1% | 5 mL |
| GlutaMAX | 1% | 5 mL |
| Pen/strep | 1% | 5 mL |
| NEAA | 1% | 5 mL |
| L-ascorbic acid | 50 μg/mL | 250 μL |
| XAV939 | 5 μM | 50 μL |
| CHIR99021 | 1 μM | 50 μL |
| A-419259 | 0.1 μM | 10 μL |
| LIF | 10 ng/mL | 50 μL |
| 2-mercaptoethanol | 10 μM | 5 μL |
4. hEPSC subculture medium
Prepare ~50 mL of bulk solution of basic medium (hEPSCM and FBS) and filter sterilize. Mix the thawed FBS by gently inverting the vial a couple of times and then aseptically transfer it to the bottle of hEPSCM. To prepare the hEPSC subculture medium, make an aliquot of the required volume for the day of hEPSCM+FBS and add Y-27632 fresh on the day of use. Filter sterilize and bring the hEPSC subculture medium solution to room temperature before use.
| Reagent | Final concentration | Volume (for 50 mL) |
|---|---|---|
| hEPSCM | 95% | 47.5 mL |
| FBS | 5% | 2.5 mL |
| Y-27632 | 10 μM | 5 μL |
5. Matrigel coating
Place the entire bottle of Matrigel in a crushed ice box, then store the ice box in a 4 °C refrigerator overnight to thaw. Ensure there is enough crushed ice to surround the bottle. After the Matrigel has melted, gently rotate the bottle to check that it has fully thawed, with no remaining ice crystals or solid clumps. Pre-cool all consumables that will come into contact with the Matrigel, such as pipette tips and tubes, and perform all procedures aseptically on ice. Dispense the Matrigel into 400 μL aliquots per tube and store at -80 °C. When needed, place the dispensed Matrigel in a crushed ice box to thaw, then transfer it to a 4 °C refrigerator overnight. Once melted, quickly transfer the Matrigel into pre-cooled sterile PBS and mix thoroughly. Matrigel-coated surfaces can be stored at 2–8 °C for up to one week.
| Reagent | Final concentration | Volume (for 40 mL) |
|---|---|---|
| PBS | 99% | 39.6 mL |
| Matrigel | 1% | 400 μL |
6. Chemically-defined basal medium (CDBM)
Prepare ~200 mL of bulk solution of basic medium (IMDM+Glu, Ham’s F12+Glu, BSA, CD lipid, ITS-X, and pen/strep) and filter sterilize. Add all ingredients to the base medium as required. Swirl the bottle to mix. CDBM can be stored at 2–8 °C for two weeks. Filter sterilize and bring the CDBM to room temperature before use.
| Reagent | Final concentration | Volume (for 200 mL) |
|---|---|---|
| IMDM+Glu | 45.25% | 90.5 mL |
| Ham’s F12+Glu | 45.25% | 90.5 mL |
| BSA | 5 mg/mL | 13.35 mL |
| CD lipid | 1% | 2 mL |
| ITS-X | 1% | 2 mL |
| Pen/strep | 1% | 2 mL |
| Monothioglycerol | 450 μM | 7.8 μL |
7. Anterior primitive streak induction medium (APSIM): 2D-d0
Prepare >40 mL of CDBM and filter sterilize. The basic medium can be stored at 2–8 °C for two weeks. To prepare APSIM, make an aliquot of the required volume for the day of basic medium solution and add Activin A, CHIR99021, and FGF2 fresh on the day of use. Filter sterilize and bring the APSIM solution to 37 °C before use.
| Reagent | Final concentration | Volume (for 40 mL) |
|---|---|---|
| CDBM | 100% | 40 mL |
| Activin A | 50 ng/mL | 20 μL |
| CHIR99021 | 10 μM | 40 μL |
| FGF2 | 20 ng/mL | 3.2 μL |
8. Paraxial mesoderm induction medium (PMIM): 2D-d1
Prepare >40 mL of CDBM and filter sterilize. The basic medium can be stored at 2–8 °C for two weeks. To prepare PMIM, make an aliquot of the required volume for the day of basic medium solution and add A8301, LDN193189, CHIR99021, and FGF2 fresh on the day of use. Filter sterilize and bring the PMIM solution to 37 °C before use.
| Reagent | Final concentration | Volume (for 40 mL) |
|---|---|---|
| CDBM | 100% | 40 mL |
| A8301 | 1 μM | 4 μL |
| LDN193189 | 250 nM | 1 μL |
| CHIR99021 | 3 μM | 12 μL |
| FGF2 | 20 ng/mL | 3.2 μL |
9. Early somite induction medium (ESIM): 2D-d2
Prepare >40 mL of CDBM and filter sterilize. The basic medium can be stored at 2–8 °C for two weeks. To prepare ESIM, make an aliquot of the required volume for the day of basic medium solution and add C59 and PD173074 fresh on the day of use. Filter sterilize and bring the ESIM solution to 37 °C before use.
| Reagent | Final concentration | Volume (for 40 mL) |
|---|---|---|
| CDBM | 100% | 40 mL |
| C59 | 1 μM | 4 μL |
| PD173074 | 100 nM | 0.4 μL |
10. Sclerotome induction medium (SCLIM): 2D-d3–d6
Prepare >40 mL of CDBM and filter sterilize. The basic medium can be stored at 2–8 °C for two weeks. To prepare SCLIM, make an aliquot of the required volume for the day of basic medium solution and add LDN193189 and SAG fresh on the day of use. Filter sterilize and bring the SCLIM solution to 37 °C before use.
| Reagent | Final concentration | Volume (for 40 mL) |
|---|---|---|
| CDBM | 100% | 40 mL |
| LDN193189 | 600 nM | 2.4 μL |
| SAG | 100 nM | 4 μL |
11. Chondrogenic basal medium (CBM)
Prepare >500 mL of the bulk solution of basic medium (DMEM/F12+Glu, ITS-X, L-ascorbic acid 2-phosphate, proline, glucose, sodium pyruvate, and pen/strep) and filter sterilize. Add all ingredients to the base medium as required. Swirl the bottle to mix. CBM can be stored at 2–8 °C for two weeks. Filter sterilize and bring the CBM to room temperature before use.
| Reagent | Final concentration | Volume (for 500 mL) |
|---|---|---|
| DMEM/F12+Glu | 95.6% | 478 mL |
| ITS-X | 1% | 5 mL |
| L-ascorbic acid 2-phosphate | 0.17 mM | 250 μL |
| Proline | 0.35 mM | 400 μL |
| Glucose | 0.15% | 1.67 mL |
| Sodium pyruvate | 1% | 5 mL |
| GlutaMAX | 1% | 5 mL |
| Pen/strep | 1% | 5 mL |
12. Early chondrocyte induction medium (ECIM): 3D-d0–d6
Prepare >50 mL of CBM and filter sterilize. The basic medium can be stored at 2–8 °C for two weeks. To prepare ECIM, make an aliquot of the required volume for the day of basic medium solution and add Dex and PDGF-BB fresh on the day of use. Filter sterilize and bring the ECIM solution to 37 °C before use.
| Reagent | Final concentration | Volume (for 50 mL) |
|---|---|---|
| CBM | 100% | 50 mL |
| Dex | 0.1 μM | 5 μL |
| PDGF-BB | 40 ng/mL | 20 μL |
13. Chondrocyte differentiation medium 1 (CDIM1): 3D-d6–d10
Prepare >50 mL of CBM and filter sterilize. The basic medium can be stored at 2–8 °C for two weeks. To prepare CDIM1, make an aliquot of the required volume for the day of basic medium solution and add Dex, PDGF-BB, and TGFβ3 fresh on the day of use. Filter sterilize and bring the CDIM1 solution to 37 °C before use.
| Reagent | Final concentration | Volume (for 50 mL) |
|---|---|---|
| CBM | 100% | 50 mL |
| Dex | 0.1 μM | 5 μL |
| PDGF-BB | 40 ng/mL | 20 μL |
| TGFβ3 | 10 ng/mL | 25 μL |
14. Chondrocyte differentiation medium 2 (CDIM2): 3D-d10–d14
Prepare >50 mL of CBM and filter sterilize. The basic medium can be stored at 2–8 °C for two weeks. To prepare CDIM2, make an aliquot of the required volume for the day of basic medium solution and add Dex, TGFβ3, and BMP4 fresh on the day of use. Filter sterilize and bring the CDIM2 solution to 37 °C before use.
| Reagent | Final concentration | Volume (for 50 mL) |
|---|---|---|
| CBM | 100% | 50 mL |
| Dex | 0.1 μM | 5 μL |
| TGFβ3 | 10 ng/mL | 25 μL |
| BMP4 | 50 ng/mL | 25 μL |
15. Chondrocyte maturation medium (CMM): 3D-d14–d28
Prepare >50 mL of CBM and filter sterilize. The basic medium can be stored at 2–8 °C for two weeks. To prepare CMM, make an aliquot of the required volume for the day of basic medium solution and add Dex, BMP4, and T3 fresh on the day of use. Filter sterilize and bring the CMM solution to 37 °C before use.
| Reagent | Final concentration | Volume (for 50 mL) |
|---|---|---|
| CBM | 100% | 50 mL |
| BMP4 | 50 ng/mL | 25 μL |
| T3 | 10 nM | 5 μL |
16. Chondrocyte hypertrophy medium (CHM): 3D-d28–d42
Prepare >50 mL of CBM and filter sterilize. The basic medium can be stored at 2–8 °C for two weeks. To prepare CHM, make an aliquot of the required volume for the day of basic medium solution and add Dex, TGFβ3, BMP4, and β-glycerophosphate fresh on the day of use. Filter sterilize and bring the CHM solution to 37 °C before use.
| Reagent | Final concentration | Volume (for 50 mL) |
|---|---|---|
| CBM | 100% | 50 mL |
| BMP4 | 50 ng/mL | 25 μL |
| T3 | 10 nM | 5 μL |
| β-glycerophosphate | 10 mM | 500 μL |
Laboratory supplies
1. 1.5 mL MaxyClear Snaplock (Axygen, catalog number: MCT-150-C)
2. Cell culture multi-well plates (12-well plate) (Corning, catalog number: 3513)
3. Cell culture multi-well plates (6-well plate) (Corning, catalog number: 3471)
4. 15 mL conical tubes (Labselect, catalog number: CT-012-15)
5. 50 mL conical tubes (Labselect, catalog number: CT-012-50)
6. 2 mL cryovials (NEST, catalog number: 607101)
7. 10 mL serological pipettes (Corning, catalog number: 4488)
8. 25 mL serological pipettes (Corning, catalog number: 4489)
9. Millex-GP syringe filter unit, pore 0.22 µm (Millipore, catalog number: SLGPR33RB)
10. 20 mL disposable sterilized syringe (Jetway, catalog number: ZSQ-20mL)
11. 10 μL pipette tips (Kirgen, catalog number: KG1011)
12. 200 µL pipette tips (Kirgen, catalog number: KG1212)
13. 1,000 µL pipette tips (Kirgen, catalog number: KG1313)
14. CountessTM cell counting chamber slides (Thermo Fisher Scientific, Invitrogen, catalog number: C10283)
15. 96-well clear round bottom ultra-low attachment microplate (Corning, catalog number: 7007)
16. 50 mL reagent reservoirs (Biosharp, catalog number: BS-PS50-RR)
Equipment
1. NordicSafe® Class II biological safety cabinet (ESCO, catalog number: NC2-L)
2. HeracellTM Vios 160i CR CO2 incubator (Thermo Fisher Scientific, catalog number: 51033773)
3. Olympus inverted fluorescence microscope (objectives: 4×, 10×, 20×) (Olympus, catalog number: IX73)
4. Laboratory centrifuge with rotors for 15 and 50 mL conical tubes and plates (Thermo Fisher Scientific, catalog number: 75007200)
5. Block M heating metal bath (FOUR E’S, catalog number: TC0401002)
6. 0.1–2.5 μL pipetter (Eppendorf, catalog number: 3123000217)
7. 0.5–10 μL pipetter (Eppendorf, catalog number: 3123000225)
8. 10–100 μL pipetter (Eppendorf, catalog number: 3123000241)
9. 20–200 μL pipetter (Eppendorf, catalog number: 3123000250)
10. 100–1000 μL pipetter (Eppendorf, catalog number: 3123000268)
11. 30–300 μL 8-channel pipette (Eppendorf, catalog number: 3123000101)
12. Mr. FrostyTM freezing container (Thermo Fisher Scientific, catalog number: 5100-0001)
13. CountessTM II FL automated cell counter (Thermo Fisher Scientific, catalog number: AMQAF1000)
14. Racks
15. Liquid nitrogen (N2) tank
16. Freezer (-20 °C)
17. Refrigerator (2–8 °C)
Procedure
文章信息
稿件历史记录
提交日期: Jan 14, 2025
接收日期: Apr 7, 2025
在线发布日期: Apr 24, 2025
出版日期: May 5, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Wang, H., Qiu, J., Lin, Y., Bai, X. and Wei, X. (2025). A Cartilaginous Organoid System Derived From Human Expanded Pluripotent Stem Cells (hEPSCs). Bio-protocol 15(9): e5304. DOI: 10.21769/BioProtoc.5304.
分类
干细胞 > 胚胎干细胞 > 细胞分化
细胞生物学 > 细胞分离和培养 > 3D细胞培养
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link