- EN - English
- CN - 中文
A Novel Optimized Silver Nitrate Staining Method for Visualizing and Quantifying the Osteocyte Lacuno-Canalicular System (LCS)
用于可视化与定量骨细胞腔道系统(LCS)的新型优化硝酸银染色方法
(*contributed equally to this work) 发布: 2025年04月20日第15卷第8期 DOI: 10.21769/BioProtoc.5289 浏览次数: 1509
评审: Vishal NehruOlga KopachAnonymous reviewer(s)

相关实验方案
采用 Davidson 固定液和黑色素漂白法优化小鼠眼组织切片的免疫组化染色
Anne Nathalie Longakit [...] Catherine D. Van Raamsdonk
2025年11月20日 1561 阅读
Abstract
The osteocyte lacuno-canalicular system (LCS) plays a crucial role in maintaining bone homeostasis and mediating cellular mechanotransduction. Current histological techniques, particularly the Ploton silver nitrate staining method, face challenges such as variations in solution concentrations and types as well as a lack of standardization, which limits their broader application in osteocyte research. In this study, we present a simplified and more effective silver nitrate staining protocol designed to address these issues. Our method utilizes a 1 mol/L silver nitrate solution combined with optimized gelatin-formic acid solutions at varying concentrations (0.05%–0.5% type-B gelatin and 0.05%–5% formic acid, or 1%–2% type-B gelatin and 0.1%–2% formic acid). Staining is performed for 1 h under 254 nm ultraviolet light or 90 min under room light, followed by washing with Milli-Q water to terminate staining. This novel optimized method yields consistent and distinct staining of the osteocyte LCS across multiple species, demonstrating superior efficiency and reliability compared to the Ploton method. It will significantly advance research in osteocyte biology and provide a valuable tool for exploring the adaptive evolution of osteocyte LCS morphology and function across various taxa.
Key features
• A novel optimized silver nitrate method using a 1 mol/L silver nitrate solution with type-B gelatin-formic acid solution effectively stains the osteocyte LCS.
• The novel optimized method is more efficient for staining the osteocyte LCS across different species.
• The novel optimized method is simpler to perform and more cost-effective than conventional methods.
Keywords: Silver Nitrate (硝酸银)Graphical overview
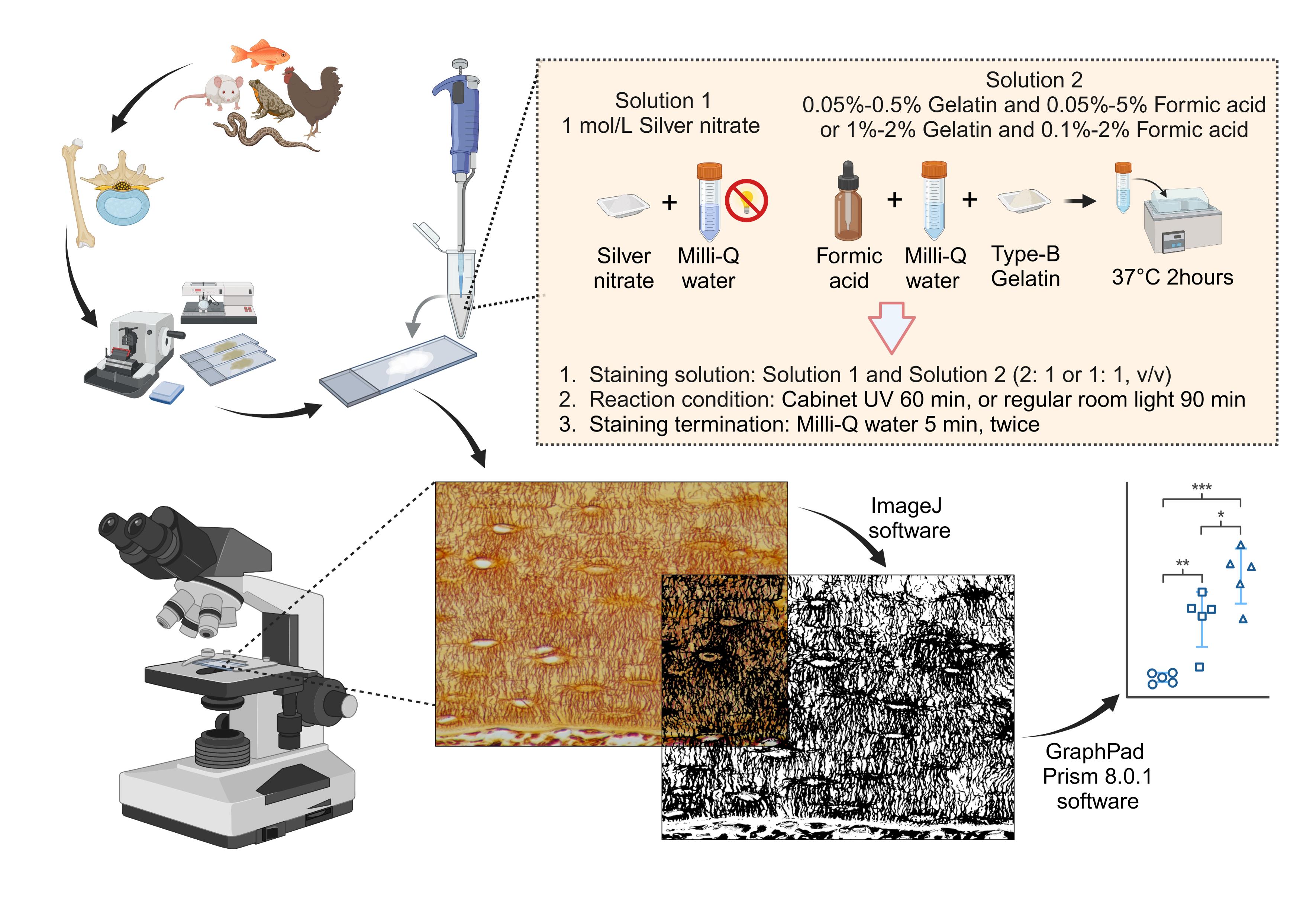
Overview of the silver nitrate staining method for staining and qualifying osteocyte lacuno-canalicular system (LCS). Created in BioRender. Libo, W. (2024) https://BioRender.com/c10z002
Background
Osteocytes and their lacuno-canalicular system (LCS) are crucial for maintaining bone homeostasis and regulating cellular mechanotransduction. The LCS facilitates nutrient exchange, waste removal, and cell signaling between osteocytes, osteoblasts, osteoclasts, and vascular endothelial cells, thereby playing a significant role in bone development and health [1–4].
The characterization and quantification of the osteocyte lacuno-canalicular system (LCS) have primarily relied on impregnation staining techniques using high concentrations of silver nitrate, particularly the 50% solution introduced by Goodpasture and Bloom in 1975 [5]. This method was adapted from earlier studies, including the work by Howell et al. on staining chromosomal nucleolus organizer regions using a 10% silver nitrate solution [6]. In 1980, Howell and Black simplified the staining procedure, developing a one-step method that improved reproducibility and ease of use [7]. In 1986, Ploton et al. modified this method by lowering the reaction temperature from 70 to 20 °C and introducing a 5% sodium thiosulfate solution to terminate the staining process, thereby enhancing the technique's overall efficiency [8]. Later, Chappard et al. applied Ploton’s method to stain nucleolus organizer regions in undecalcified, methyl methacrylate (MMA)-embedded bones, inadvertently discovering its effectiveness for staining the osteocyte LCS [9–10]. They also established optimal staining conditions as 55 min at room temperature in the dark, which have since become the standard method [11–12].
By the early 2000s, the importance of osteocytes and LCS in bone remodeling had gained widespread attention. Researchers began adopting these staining solutions and protocols to study osteocyte biology. This method, commonly referred to as the Ploton silver nitrate staining method, facilitated significant advancements in understanding the role of osteocytes in bone health and disease [13–16]. Despite its widespread use, the Ploton method is influenced by various factors—such as silver nitrate concentration, gelatin-formic acid concentration, gelatin type, lighting conditions, and termination methods—that can result in unclear staining backgrounds, as shown in some studies [17–18]. These variables can hinder the accurate quantitative analysis of the osteocyte LCS.
In this paper, we present a novel optimized method that uses a 1 mol/L silver nitrate solution combined with a gelatin-formic acid solution at varying concentrations (0.05%–0.5% type-B gelatin and 0.05%–5% formic acid, or 1%–2% type-B gelatin and 0.1%–2% formic acid). The staining process is carried out under 254 nm ultraviolet light for 60 min or under regular room light for 90 min, followed by termination of the reaction with Milli-Q water. This novel optimized method enhances the efficiency of the staining process and effectively stains the osteocyte LCS across different vertebrate taxa. It offers a valuable tool for studies of osteocyte biology and LCS morphogenesis, providing new insights into the role of osteocytes in bone biology.
Materials and reagents
Biological materials
1. 8-week-old male C57BL/6J mice and 8-week-old Sprague-Dawley rats were obtained from Hangzhou Ziyuan Laboratory Animal Technology Co., Ltd., Hangzhou, China
2. Rabbit (Oryctolagus cuniculus), silver carp (Hypophthalmichthys sp.), common carp (Cyprinus carpio), Tai Lake white fish (Anabarilius sp.), bullfrogs (Lithobates catesbeiana), chickens (Gallus gallus domesticus), and wall lizards (Gekko sp.) were obtained from a local food market and an online traditional Chinese medicine herb store
Reagents
1. 10% EDTA decalcifying solution (Sangon Biotech, catalog number: E671001–0500)
2. Sodium chloride (Sangon Biotech, catalog number: A610476)
3. Potassium chloride (Sangon Biotech, catalog number: A610440)
4. Sodium phosphate dibasic (Sangon Biotech, catalog number: A501727)
5. Potassium dihydrogen phosphate (Sangon Biotech, catalog number: A600445)
6. Silver nitrate (Sangon Biotech, catalog number: C510027–0010)
7. 10% neutral buffered formalin (Wexis, catalog number: 311010014)
8. Paraffin (Leica, catalog number: 39601006)
9. Ethanol (Sinopharm, catalog number: 10009218)
10. Xylene (Sinopharm, catalog number: 10023418)
11. 88% formic acid (Sinopharm, catalog number: 10010118)
12. Type-B gelatin (Solarbio, catalog number: G8061)
13. Neutral balsam mounting medium (Solarbio, catalog number: G8590)
14. Cedar oil (Sangon Biotech, catalog number: A502219–0025)
Solutions
1. 1 mol/L silver nitrate solution (see Recipes)
2. 1% (v/v) formic acid solution (see Recipes)
3. 2% (w/v) type-B gelatin in 1% formic acid solution (see Recipes)
4. Staining solution (see Recipes)
5. PBS solution (0.01 mol/L, pH 7.4) (see Recipes)
Recipes
1. 1 mol/L silver nitrate solution
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Silver nitrate powder | 1 mol/L | 8.75 g |
| Milli-Q water (18.2 MΩ·cm) | n/a | 50 mL |
| Total | n/a | 50 mL |
Prepare the solution and store it at room temperature, protected from light. You can adjust the volume up or down depending on the number of slides to be stained. You can also purchase the pre-prepared 1 mol/L silver nitrate solution online, which is readily available and commonly used in chemical titration experiments.
2. 1% (v/v) formic acid solution
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Formic acid (88%) | 1% (v/v) | 0.568 mL |
| Milli-Q water (18.2 MΩ·cm) | n/a | 49.432 mL |
| Total | n/a | 50 mL |
Store the solution at room temperature. You can adjust the volume up or down depending on the number of slides to be stained.
3. 2% (w/v) type-B gelatin in 1% formic acid solution
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Type-B gelatin | 0.02 g/mL | 1 g |
| 1% formic acid solution | n/a | 50 mL |
| Total | n/a | 50 mL |
Dissolve the mixture by warming it in a 37 °C water bath for approximately 2 h. Once the mixture has fully dissolved, it can be stored at room temperature. You can adjust the volume up or down depending on the number of slides to be stained. Based on our experience, a gelatin-formic acid solution can be prepared using either 0.05%–1% (w/v) type-B gelatin and 0.05%–5% (v/v) formic acid or 1%–2% (w/v) type-B gelatin and 0.1%–2% (v/v) formic acid. This formulation yields staining results as clear and effective as those obtained with a 2% (w/v) type-B gelatin in 1% formic acid solution.
4. Staining solution
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| 1 mol/L silver nitrate solution | 2/3 or 33.3% (v/v) | 0.2 mL |
| 2% type-B gelatin in 1% formic acid solution | 1/3 or 66.7% (v/v) | 0.1 mL |
| Total | n/a | 0.3 mL |
Prepare the staining solution immediately before use, ensuring it is kept away from light. This should be done after washing the tissue sections with Milli-Q water and applying the solution to the bone tissue on the slide without delay. Avoid preparing the solution in advance, as this may lead to poor or incomplete staining results. The volume ratio of silver nitrate solution to 2% gelatin and 1% formic acid solution can be either 2:1 or 1:1. Based on our experience, 0.3 mL of staining solution is sufficient for thorough staining of a slide with a single bone tissue section attached.
5. PBS solution (0.01 mol/L, pH 7.4)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Sodium chloride | 0.137 mol/L | 8.006 g |
| Potassium chloride | 2.7 mmol/L | 0.201 g |
| Sodium phosphate dibasic | 1.8 mmol/L | 1.419 g |
| Potassium dihydrogen phosphate | 0.01 mol/L | 0.244 g |
| Milli-Q water (18.2 MΩ·cm) | n/a | 1,000 mL |
| Total | n/a | 1,000 mL |
Laboratory supplies
1. 1.5 mL microcentrifuge tubes (Axygen, catalog number: MCT-150-C)
2. Microscope slides (Citotest, catalog number: 80312–3161)
3. Microscope cover slides (Citotest, catalog number: 80340–0130)
4. Pipettes (Gilson, model: P1000 and P200)
5. 50 mL tubes (Greiner, catalog number:227651)
6. Embedding cassettes (Citotest, catalog number: 31050102W)
7. Forceps and scissors
Equipment
1. Biological safety cabinet (Thermo Fisher Scientific, model: 1379/1389 with 254 nm UV light G3675L 31~40 W)
2. Automatic benchtop tissue processor (Leica, model: TP1020)
3. Tissue embedding station (Leica, model: EG1160)
4. Rotary microtomes (Leica, model: RM2135)
5. Histology water bath (Leica, model: Histobath HI1210)
6. Ultrapure water purification system (Millipore, model: Milli-Q Elix® Essential)
7. Water bath (JingHong, model: DNB-501S)
8. Thermoelectric oven (JingHong, model: DNP-9052)
9. Analytical balance (Sartorius, model: BSA124S)
10. Nikon brightfield microscope (Nikon, model: ECLIPSE 200)
Software and datasets
1. ImageJ software (NIH, USA)
2. GraphPad Prism 8.0.1
3. BioRender (https://www.biorender.com/)
Procedure
文章信息
稿件历史记录
提交日期: Jan 1, 2025
接收日期: Mar 25, 2025
在线发布日期: Apr 7, 2025
出版日期: Apr 20, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/).
如何引用
Wu, J., Xue, C., Li, Q., Wu, H., Zhang, J., Wang, C., Dai, W. and Wang, L. (2025). A Novel Optimized Silver Nitrate Staining Method for Visualizing and Quantifying the Osteocyte Lacuno-Canalicular System (LCS). Bio-protocol 15(8): e5289. DOI: 10.21769/BioProtoc.5289.
分类
细胞生物学 > 细胞染色 > 全细胞
细胞生物学 > 组织分析 > 组织成像
细胞生物学 > 细胞结构
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link