- EN - English
- CN - 中文
Antibody Purification Using Spy Chemistry
使用 Spy Chemistry 进行抗体纯化
发布: 2025年04月20日第15卷第8期 DOI: 10.21769/BioProtoc.5286 浏览次数: 1882
评审: Alessandro DidonnaDawid S ZylaCatherine Hurd

相关实验方案
蛋白质pull-down免疫共沉淀实验分析病毒DNA结合蛋白质之间的直接相互作用
Ana Lechuga [...] Modesto Redrejo-Rodríguez
2018年01月05日 12430 阅读
采用Förster共振能量转移法检测大肠埃希杆菌细胞质和周质中蛋白质的相互作用
Nils Y. Meiresonne [...] Tanneke den Blaauwen
2018年01月20日 14444 阅读
基于蛋白筛选策略从合成文库中分离抗原特异性纳米抗体:结合 MACS 的酵母展示筛选与 FLI-TRAP 方法
Apisitt Thaiprayoon [...] Dujduan Waraho-Zhmayev
2026年01月20日 401 阅读
Abstract
Antibody purification is a fundamental technology for therapeutic and diagnostic applications. While traditional methods like ammonium sulfate precipitation and polyethylene glycol precipitation are cost-effective, they often result in low purity and require multiple purification steps. Protein A–based affinity chromatography, the gold standard for antibody purification, provides high specificity but can be further improved to increase loading capacity and reduce costs. In this protocol, we introduce a novel approach for purifying high-quality, high-purity antibodies from complex samples using SpyFixer/Z domain–modified resin. This method utilizes Spy chemistry for site-specific immobilization of the Z domain of Protein A, significantly enhancing antibody loading capacity up to 200 mg/mL resin and ensuring stable, durable immobilization. Using this protocol, we achieved >90% purity of human immunoglobulin G (hIgG) from diverse sources, including E. coli cell lysates, human serum, and industrial fermentation broth. The SpyFixer/Z domain–modified resin protocol is simple, cost-effective, and offers a robust, scalable solution for efficient antibody purification in bioengineering applications. This immobilization scheme based on Spy chemistry can also be extended to other immunoglobulin-binding proteins, such as Protein G and Protein L, to develop high-efficiency affinity resins.
Key features
• This protocol builds upon purification methods developed by Lin’s lab [1], providing more detailed steps than the previously published study.
• The protocol offers a useful and standardized approach for purifying antibodies and Fc-fusion proteins.
• The SpyFixer/Z domain–modified resin is easy to prepare, reusable, and offers a cost-effective solution.
Keywords: Antibody purificationGraphical overview
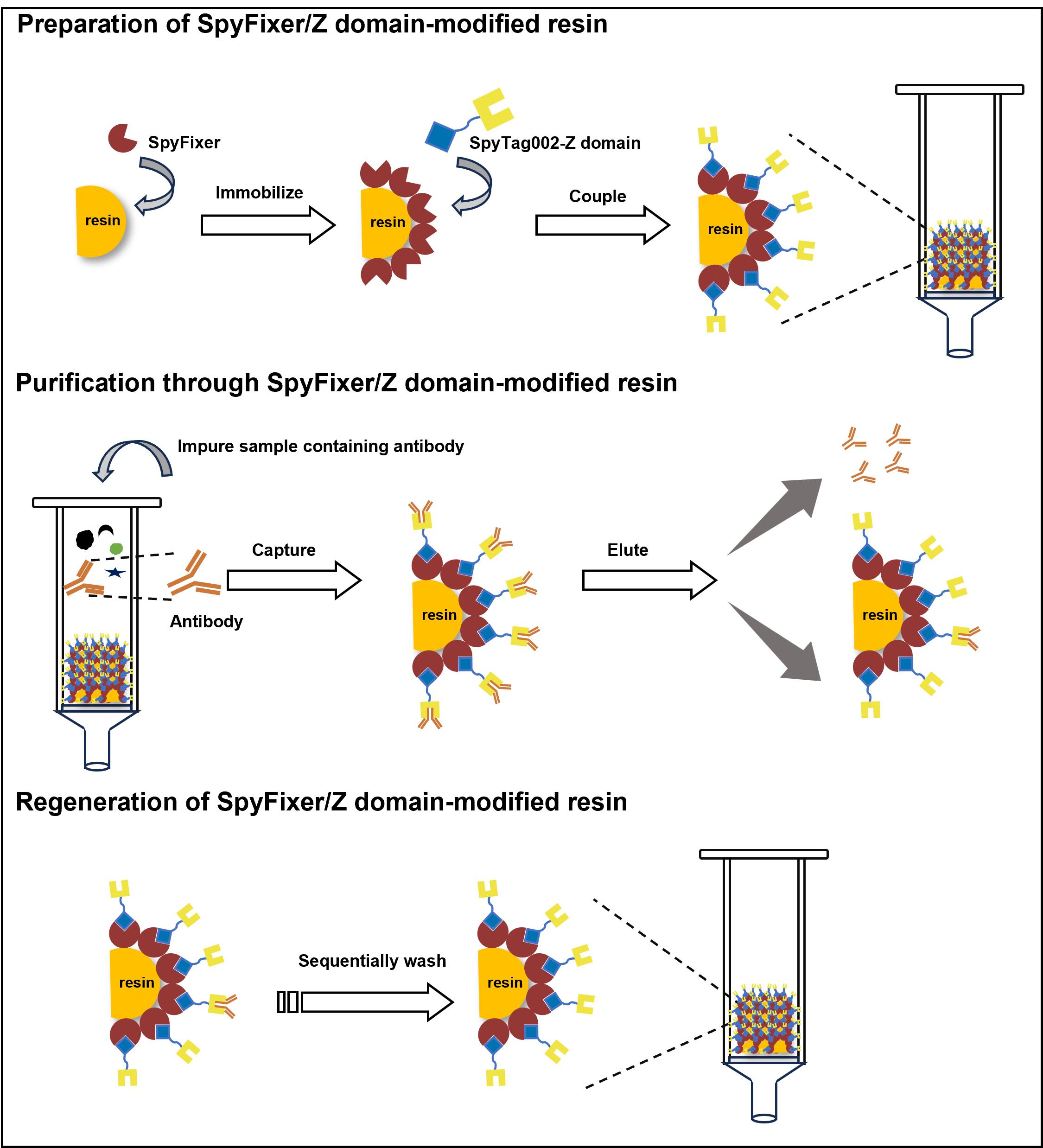
Background
Antibodies are indispensable in modern medicine, playing a crucial role in therapeutic and diagnostic applications. The global monoclonal antibody (mAb) market has experienced rapid growth, projected to reach USD 263.28 billion in 2024 and USD 792.37 billion by 2032 [2]. To date, more than 150 recombinant antibody-based drugs have been approved by the FDA for the treatment of various diseases, such as cancer, rheumatic diseases, and infectious diseases [3]. As production cost pressures shift from fermentation to downstream processing, purification has become a significant bottleneck in antibody manufacturing.
Traditional purification methods such as ammonium sulfate precipitation and polyethylene glycol (PEG) precipitation are cost-effective but often result in co-precipitation with impurities, leading to insufficient purity and requiring multiple purification steps [4,5]. In contrast, Staphylococcal Protein A–based affinity chromatography has emerged as the gold standard for antibody purification, relying on the specific interaction between the immunoglobulin G (IgG) Fc region and immobilized Protein A ligands [6]. Most antibody fragments typically lack an Fc domain and do not bind to Protein A resins with high affinity [7]. Alternative immunoglobulin-binding proteins, such as Protein G (30 kDa) and Protein L (76–106 kDa), offer affinity-based purification strategies for antibody fragments, including antigen-binding fragments (Fabs), single-chain variable fragments (scFvs), and single-domain antibody fragments (dAbs), which typically lack an Fc domain and do not effectively bind to Protein A resins [8]. The use of Protein G involves low pH elution conditions and higher cost compared to Protein A resins [9,10]. Protein L binds specifically to antibodies containing certain kappa light chain subtypes, but this specificity limits its application in monoclonal antibody purification processes [6,9].
As an alternative affinity ligand, the Z domain—derived from the B domain of Protein A—has garnered attention for its advantages over full-length Protein A (~42 kDa), including its smaller size (~6.6 kDa), ease of engineering, higher stability, and comparable affinity [11,12]. These properties make the Z domain a promising candidate for antibody affinity purification. Additionally, the SpyTag/SpyCatcher system, a peptide-protein pair that spontaneously forms a covalently linked complex, has demonstrated significant potential for site-specific ligand immobilization [13]. To enhance immobilization efficiency and retention of binding activity, we employed SpyFixer, a variant of SpyCatcher, to site-specifically immobilize the Z domain onto a resin, generating SpyFixer/Z domain–modified resin [1].
Here, we develop an advanced antibody purification protocol using SpyFixer/Z domain–modified resin. This method achieves >90% purity and reusability, comparable to conventional Protein A–based methods while offering several advantages such as the use of inexpensive epoxy resins and increased antibody loading capacity [1]. These improvements provide a more cost-effective and scalable solution for antibody purification, with broad applications in biotechnology and pharmaceuticals. Using this protocol, we successfully purified IgG directly from complex samples, including E. coli cell lysates, human serum, and industrial fermentation broth [1].
Materials and reagents
Biological materials
1. E. coli BL21(DE3) competent cells (TransGen Biotech, catalog number: CD901-02)
2. Human immunoglobulin G (hIgG) (Sigma-Aldrich, catalog number: I4506)
Reagents
1. Bovine serum albumin (BSA) (Sigma-Aldrich, catalog number: V900933)
2. 6× Protein loading buffer (TransGen Biotech, catalog number: DL101-02)
3. Premixed protein marker (Takara, catalog number: 3595A)
3. Sodium chloride (NaCl) (Damao Chemical Reagent Factory, CAS number: P000967)
4. Tryptone (Oxoid, catalog number: LPOO24)
5. Yeast extract (Oxoid, catalog number: LP0021)
6. Kanamycin (Aladdin, catalog number: K103024)
7. Ampicillin (Aladdin, catalog number: A102050)
8. Isopropyl-β-D-1-thiogalactopyranoside (IPTG) (Sangon Biotech, catalog number: A100487-0025)
9. Tris(2-carboxyethyl)phosphine (TCEP) (Thermo Fisher Scientific, catalog number: T2556)
10. Sodium dihydrogen phosphate (NaH2PO4) (Sangon Biotech, catalog number: A501726)
11. Disodium hydrogen phosphate (Na2HPO4) (Sangon Biotech, catalog number: A501727)
12. Potassium dihydrogen phosphate (KH2PO4) (Sangon Biotech, catalog number: A600445)
13. Imidazole (Sigma-Aldrich, catalog number: V900153)
14. Potassium chloride (KCl) (Aladdin, catalog number: P112134)
15. Sodium sulfate (Na2SO4) (Sigma-Aldrich, catalog number: 238597)
16. Ethanolamine (Aladdin, catalog number: E103810)
17. Acetic acid (Sangon Biotech, catalog number: A501931)
18. Sodium hydroxide (NaOH) pellets (Damao Chemical Reagent Factory, catalog number: P000880)
19. Hydrochloric acid (HCl) (Cologne Chemical Reagent Factory, catalog number: LC149502)
20. Acetic acid (Sangon Biotech, catalog number: A501931)
21. Tris-base (Biofroxx, catalog number: 1115GR500)
22. Arginine (Sangon Biotech, catalog number: A600205)
23. Guanidine hydrochloride (GdnHCl) (Aladdin, catalog number: G108676)
24. Ethanol (EtOH) (Damao Chemical Reagent Factory, CAS number: 64-17-5)
25. YoungPAGETM MES SDS running buffer powder (GenScript, catalog number: M00677)
26. SOC medium (Sangon Biotech, catalog number: B540119)
Solutions
1. LB medium (see Recipes)
2. Kanamycin stock (see Recipes)
3. Ampicillin stock (see Recipes)
4. 1 M IPTG (see Recipes)
5. Binding buffer (see Recipes)
6. Elution buffer (see Recipes)
7. 100 mM PBS (see Recipes)
8. 1 M NaH2PO4 (see Recipes)
9. 1 M Na2HPO4 (see Recipes)
10. Coupling buffer (see Recipes)
11. 1 M ethanolamine (see Recipes)
12. 0.1 M acetate buffer (see Recipes)
13. 0.1 M Tris-HCl buffer (see Recipes)
14. 0.5 M arginine solution (see Recipes)
15. 6 M GdnHCl (see Recipes)
16. 0.1 M NaOH (see Recipes)
17. 20% ethanol (see Recipes)
Recipes
1. LB medium
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| NaCl | 10 g/L | 10 g |
| Tryptone | 10 g/L | 10 g |
| Yeast extract | 5 g/L | 5 g |
| Double-distilled water (ddH2O) | n/a | To 1,000 mL |
| Total | n/a | 1,000 mL |
Sterilize using an autoclave at 121 °C for 15 min.
2. Kanamycin stock
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Kanamycin | 50 mg/mL | 0.5 g |
| ddH2O | n/a | To 10 mL |
| Total | n/a | 10 mL |
Sterilize by filtering with a 0.22 μm syringe filter. Store in 1 mL aliquots at -20 °C.
3. Ampicillin stock
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Ampicillin | 100 mg/mL | 1 g |
| ddH2O | n/a | To 10 mL |
| Total | n/a | 10 mL |
Sterilize by filtering with a 0.22 μm syringe filter. Store in 1 mL aliquots at -20 °C.
4. 1 M IPTG
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| IPTG | 1 M | 2.38 g |
| ddH2O | n/a | To 10 mL |
| Total | n/a | 10 mL |
Sterilize by filtering with a 0.22 μm syringe filter. Store in 1 mL aliquots at -20 °C.
5. Binding buffer (pH 7.4)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Na2HPO4 | 15.6 mM | 2.22 g |
| KH2PO4 | 4.4 mM | 0.6 g |
| NaCl | 500 mM | 29.22 g |
| Imidazole | 30 mM | 2.04 g |
| ddH2O | n/a | To 1,000 mL |
| Total | n/a | 1,000 mL |
Adjust pH to 7.4 with 12 M HCl. Filter through a 0.22 μm hydrophilic membrane.
6. Elution buffer (pH 7.4)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Na2HPO4 | 15.6 mM | 2.22 g |
| KH2PO4 | 4.4 mM | 0.6 g |
| NaCl | 500 mM | 29.22 g |
| Imidazole | 500 mM | 34.04 g |
| ddH2O | n/a | To 1,000 mL |
| Total | n/a | 1,000 mL |
Adjust pH to 7.4 with 12 M HCl. Filter through a 0.22 μm hydrophilic membrane.
7. 100 mM PBS (pH 7.0)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Na2HPO4 | 94.7 mM | 13.44 g |
| KH2PO4 | 5.3 mM | 0.72 g |
| NaCl | 137 mM | 8 g |
| KCl | 2.7 mM | 0.2 g |
| ddH2O | n/a | To 1,000 mL |
| Total | n/a | 1,000 mL |
Adjust pH to 7.0 with 12 M HCl. Filter through a 0.22 μm hydrophilic membrane.
8. 1 M NaH2PO4
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| NaH2PO4 | 1 M | 59.99 g |
| ddH2O | n/a | To 500 mL |
| Total | n/a | 500 mL |
9. 1 M Na2HPO4
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Na2HPO4 | 1 M | 70.98 g |
| ddH2O | n/a | To 500 mL |
| Total | n/a | 500 mL |
10. Coupling buffer (pH 10.0)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Na2SO4 | 250 mM | 35.51 g |
| 1 M NaH2PO4 | 6.8 mM | 6.8 mL |
| 1 M Na2HPO4 | 93.2 mM | 93.2 mL |
| ddH2O | n/a | To 1,000 mL |
| Total | n/a | 1,000 mL |
Adjust pH to 8.0 with extra 1 M Na2HPO4. Adjust pH from 8.0 to 10.0 with 1 M NaOH. Filter through a 0.22 μm hydrophilic membrane.
11. 1 M ethanolamine (pH 8.0)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Ethanolamine | 1 M | 12.1 mL |
| ddH2O | n/a | To 200 mL |
| Total | n/a | 200 mL |
Adjust pH to 8.0 with 12 M HCl. Filter through a 0.22 μm hydrophilic membrane.
12. 0.1 M acetate buffer (pH 4.0)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Acetic acid | 0.1 M | 571.9 μL |
| NaCl | 0.5 M | 2.92 g |
| ddH2O | n/a | To 100 mL |
| Total | n/a | 100 mL |
Adjust pH to 4.0 with 1 M NaOH. Filter through a 0.22 μm hydrophilic membrane.
13. 0.1 M Tris-HCl buffer (pH 8.0)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Tris-Base | 0.1 M | 1.21 g |
| NaCl | 0.5 M | 2.92 g |
| ddH2O | n/a | To 100 mL |
| Total | n/a | 100 mL |
Adjust pH to 8.0 with 12 M HCl. Filter through a 0.22 µm hydrophilic membrane.
14. 0.5 M arginine solution (pH 3.8)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Arginine | 0.5 M | 17.42 g |
| ddH2O | n/a | To 200 mL |
| Total | n/a | 200 mL |
Adjust pH to 3.8 with 12 M HCl. Filter through a 0.22 μm hydrophilic membrane.
15. 6 M GdnHCl (pH 2.0)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Guanidine hydrochloride | 6 M | 289.5 g |
| ddH2O | n/a | To 500 mL |
| Total | n/a | 500 mL |
Adjust pH to 2.0 with 12 M HCl. Filter through a 0.22 μm hydrophilic membrane.
16. 0.1 M NaOH
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| NaOH | 0.1 M | 2 g |
| H2O | n/a | To 500 mL |
| Total | n/a | 500 mL |
Filter through a 0.22 μm hydrophilic membrane.
17. 20% ethanol
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Ethanol | 20% (v/v) | 200 mL |
| ddH2O | n/a | To 1,000 mL |
| Total | n/a | 1,000 mL |
Filter through a 0.22 μm hydrophilic membrane.
Laboratory supplies
1. HisTrapTM HP column (Cytiva, catalog number: 17524802)
2. Epoxy-activated Seplife® FF resin (Sunresin, catalog number: A40309010)
3. Spectrophotometer cuvettes (Sangon, catalog number: F511002-0001)
4. Empty spin columns (Biocomma, catalog number: 007400)
5. 1 mL empty chromatography column (NanoMicro, catalog number: 10000077022X)
6. 0.2 mL PCR tubes (Axygen, catalog number: PCR-2CP-RT-C)
7. 1.5 mL microcentrifuge tubes (Axygen, catalog number: MCT-150-C)
8. 2 mL microcentrifuge tubes (Axygen, catalog number: MCT-200-C)
9. 15 mL centrifuge tubes (Corning, catalog number: 430791)
10. 50 mL centrifuge tubes (Corning, catalog number: 430829)
11 0.22 μm syringe filters (Pall Corporation, catalog number: 4652)
12. 20 mL syringes (double-dove, catalog number: HX-SLZSQ-SG-20ML/16)
13. 0.22 μm hydrophilic membrane filter (Millipore Sigma, catalog number: GSWP04700)
14. Amicon Ultra centrifugal filter units (3 K MWCO) (Millipore Sigma, catalog number: UFC900396)
15. Amicon Ultra centrifugal filter units (10 K MWCO) (Millipore Sigma, catalog number: UFC901096)
16. YoungPAGETM Precast gel (GenScript, catalog number: M00928)
17. SnakeSkinTM dialysis tubing, 3.5 K MWCO (Thermo Fisher Scientific, catalog number: 88244)
Equipment
1. Pipetting system (Gilson, model: PIPETMAN Classic Starter Kit)
2. Shaking incubator (New Brunswick Scientific, model: Innova44)
3. Biochemical incubator (Thermo Fisher Scientific, model: IMH100-S)
4. Spectrophotometer (Youke Instrument, model: UV755B)
5. Electronic analytical balance (Mettler Toledo, model: MS204TS)
6. Autoclave (Hirayama, model: HVE-50)
7. -80 °C freezer (Thermo Fisher Scientific, model: 995)
8. Clean bench (Donglian, model: DL-CJ-2NDII)
9. Ultrasonic homogenizer (Scientz, model: JY92-)
10. Centrifuge for 15 and 50 mL tubes (Eppendorf, model: 5810R)
11. Centrifuge for 1.5 and 2 mL tubes (Eppendorf, model: 5425R)
12. Electrophoresis equipment (Bio-Rad, model: Mini-PROTEAN® Tetra Cell Systems)
13. pH meter (Mettler Toledo, model: FE28-standard)
14. Mini rotator (Gilson, model: Roto-MiNi Plus)
15. AKTA Purifier chromatography system (GE Healthcare, model: AKTA Purifier 10)
16. Protein staining instrument (GenScript, model: eStain L1)
17. Vacuum filtration system (Ilmvac GmbH, model: 120788)
18. Dry bath incubator (Coyote, model: H2O3-100C)
Software and datasets
1. SnapGene® (SnapGene, https://www.snapgene.com/)
2. ImageJ (National Institutes of Health DNA, https://imagej.net/ij/)
3. Unicorn for AKTA purifier (GE Healthcare, https://www.cytivalifesciences.com/en/us/shop/chromatography/software/unicorn-7-p-05649)
Procedure
文章信息
稿件历史记录
提交日期: Jan 21, 2025
接收日期: Mar 19, 2025
在线发布日期: Apr 3, 2025
出版日期: Apr 20, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Yang, X., Lin, Z., Xiang, Y. and Lao, Z. (2025). Antibody Purification Using Spy Chemistry. Bio-protocol 15(8): e5286. DOI: 10.21769/BioProtoc.5286.
分类
生物化学 > 蛋白质 > 分离和纯化
生物化学 > 蛋白质 > 相互作用 > 蛋白质-蛋白质相互作用
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link









