- EN - English
- CN - 中文
Endo-1,4-β-D-xylanase Assay Using Azo-Xylan and Variants Thereof
基于Azo-Xylan及其变体的Endo-1,4-β-D-木聚糖酶活性检测方法
(*contributed equally to this work) 发布: 2025年04月20日第15卷第8期 DOI: 10.21769/BioProtoc.5283 浏览次数: 1914
评审: Alba BlesaAnonymous reviewer(s)
Abstract
Xylan is the main component of hemicellulose and consists of a complex heteropolysaccharide with a heterogeneous structure. This framework, in addition to the crystalline structure of cellulosic fibers and the rigidity of lignin, makes lignocellulosic biomass (LCB) highly recalcitrant to degradation. Xylanases are glycoside hydrolases that cleave the β-1,4-glycoside linkages in the xylan backbone and have attracted increasing attention due to their potential uses in various industrial sectors such as pulp and paper, baking, pharmaceuticals, and lignocellulosic biorefining. For decades, the measurement of xylanase activity was based on reducing sugar quantification methods like DNS or Nelson/Somogyi assays, with numerous limitations in terms of specificity and interference from other enzymatic activities. A better alternative is the colorimetric Azo-Xylan assay, which specifically measures the endo-1,4-β-D-xylanase activity. In this study, the Azo-Xylan protocol was adapted from the company Megazyme to determine the enzymatic activity of thermostable xylanases produced by microbial consortia (i.e., microbiomes), aiming to determine biochemical features such as temperature and pH optima, thermostability, and shelf life. This modified approach offers a rapid, cost-effective, and highly specific method for the determination of xylanase activity in complex mixtures, helping the development of a xylanase-based method for the hydrolysis of hard-degrading substrates in bio-based industries.
Key features
• Direct enzyme assay for qualitative xylanase activity detection or quantitative measurement with a calibration curve.
• Specific for determination of endo-1,4-β-D-xylanase activity, allowing to overcome interferences by enzymes with other activities.
Keywords: Xylanases (木聚糖酶)Graphical overview
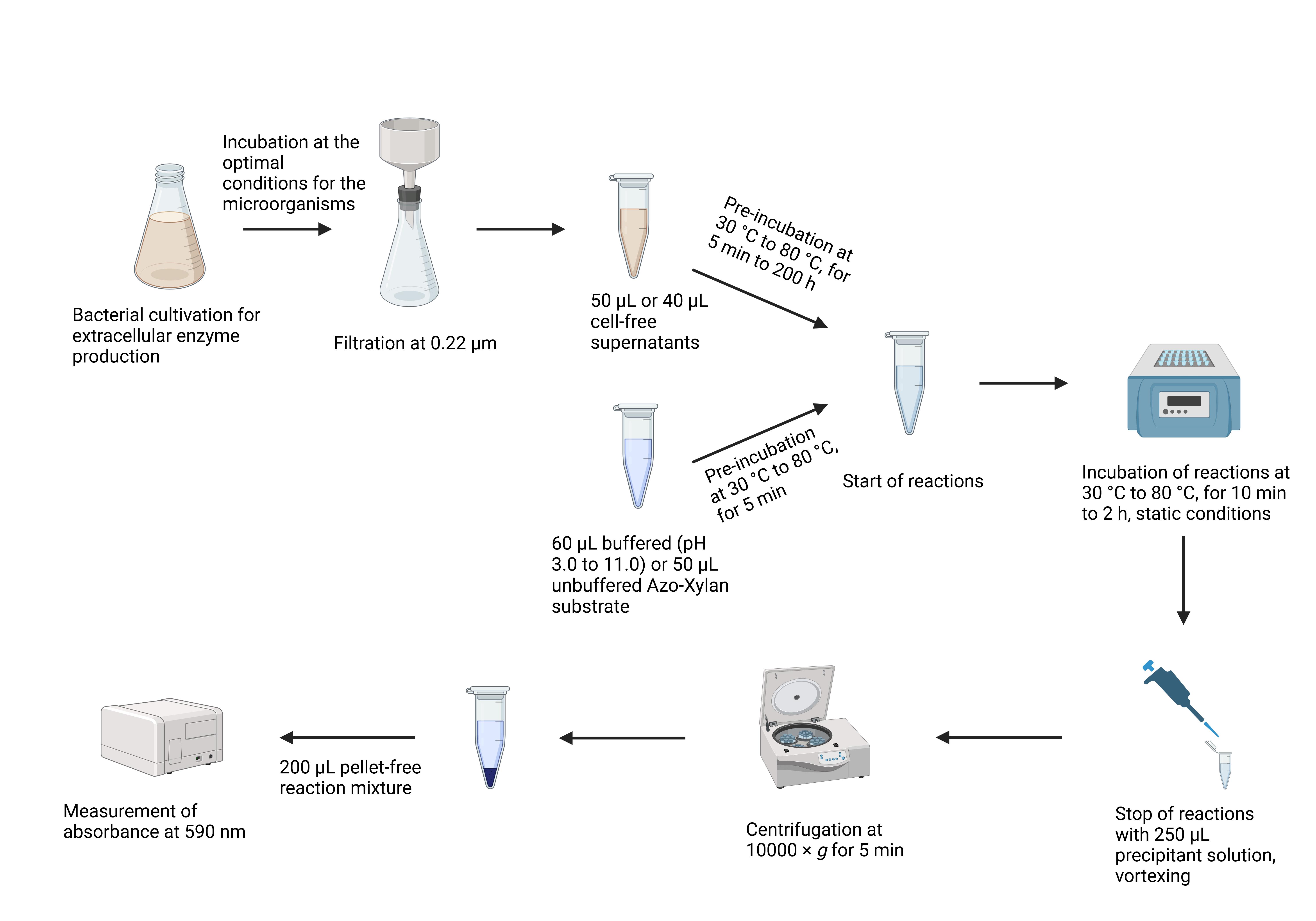
Key steps of the Azo-Xylan assay
Background
Xylan is one of the most abundant polysaccharides in nature, having a highly complex tertiary structure organized as a linear backbone of D-xylose with β-1,4-linkages, usually branched with other sugars such as α-arabinose and/or organic acids [1,2]. Due to its heterogeneous architecture, xylan degradation is very challenging, but the process can be effectively carried out by xylanases that hydrolyze β-1,4-glycosidic bonds. Xylanases are glycoside hydrolases (GHs) expressed by various organisms, including bacteria, yeast, fungi, and crustaceans [3]. The genomic, structural, and functional information of xylanase can be found in the carbohydrate-active enzyme database (CAZy) [4]. Xylanases are generally associated with GH families 5, 7, 8, 9, 10, 11, 12, 16, 26, 30, 43, 44, 51, and 62 [4], hydrolyzing xylan substrates according to two modes of action: retention and inversion [5,6]. This wide spectrum of physicochemical properties, substrate specificities, and catalytic mechanisms makes xylanases highly versatile for numerous applications across industrial and biotechnological sectors [7]. Nowadays, their use spans pulp bleaching in pulp and paper industries [8], bakery and food processing industries [9,10], prebiotics and potential anticancer agents in pharmaceutical sectors [11,12], and lignocellulosic biorefining [13].
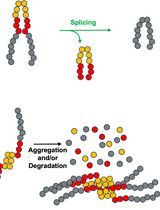
Numerous methods have been developed to measure endo-xylanase activity. These assays differ not only in assay conditions (temperature, incubation time, substrate, etc.) but also in the quantification method of enzymatic activity. For decades, the preferred method was the measurement of reducing sugars liberated from the polymer through the 3,5-dinitrosalicylic acid (DNS) method [14] or the Nelson/Somogyi method [15,16]. However, both procedures present some limitations, especially in terms of a lack of stoichiometric relation of the color response and turbidity [17]; in addition, these assays are nonspecific, and other enzymatic activities can be involved in the product determination, such as β-xylosidase and α-glucuronidase [18]. Viscosimetric methods provide benefits in terms of specificity and sensitivity, but they are also time-consuming and show a limited throughput of just a few reactions per day [18]. Nevertheless, fermentation broths or complex mixtures are generally composed of a cocktail of endo-xylanase and other glycosidases, including α-L-arabinofuranosidase and β-xylosidase, making the process of quantification quite challenging. To overcome these limitations, soluble dyed polysaccharides, such as Azo-Xylan or Azo-wheat arabinoxylan, are extensively used and give the possibility to measure enzymatic activity even in preparations with high levels of reducing sugars [18]. The Azo-Xylan assay is specific for endo-1,4-β-D-xylanase activity, and the protocol can be carried out following the manufacturer’s instructions (Megazyme, Bray, Ireland). In principle, to obtain Azo-Xylan, birchwood xylan is first purified to remove starch and then it is chemically dyed with Remazol Brilliant Blue R™ to an extent of approximately one dye molecule per 30 sugar residues. After incubation of Azo-Xylan with endo-xylanases, the substrate is depolymerized to produce low-molecular-weight water-soluble fragments (Figure 1), which remain in solution, resulting in a colored supernatant that can be directly correlated to endo-xylanase activity by reference to a standard curve (Megazyme, Bray, Ireland). Herein, we adapted the classical version of this protocol to measure the endo-xylanase activity of thermostable xylanases secreted by different microbial consortia, aiming to the determination of enzyme temperature and pH optima, thermostability, and shelf life [19,20]. The modifications can help in the determination of the biochemical parameters of enzymes in complex mixtures and can enhance the possibility of obtaining a complete characterization in a rapid and cheap way.
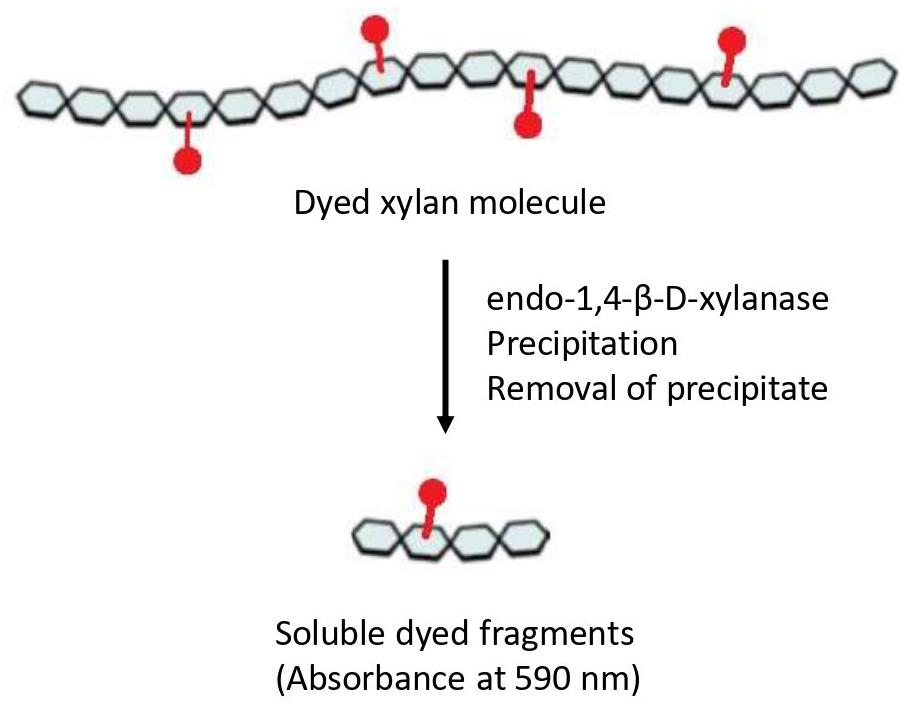
Figure 1. Representation of the Azo-Xylan molecule. Adapted from the picture available at Megazyme website (https://prod-media.megazyme.com/media/pdf/71/fd/ce/enzyme-substrates-brochure.pdf).
Materials and reagents
Biological materials
1. Cell-free supernatants (CFSs), mixture of extracellularly secreted enzymes obtained from filtration of microbial culture broth at 0.22 μm
Reagents
1. Azo-Xylan from Birchwood (Megazyme, catalog number: S-AXBP)
3. Ethanol (Honeywell, catalog number: 02870)
4. Citric acid (Merck, catalog number: 1.00244.1000)
5. Sodium dihydrogen phosphate (NaH2PO4) (Chemlab, catalog number: CL00.1497.1000)
6. Disodium hydrogen phosphate (Na2HPO4) (Chemlab, catalog number: CL00.1463.1000)
7. Potassium dihydrogen phosphate (KH2PO4) (Sigma-Aldrich, catalog number: P5655)
8. Tris (Neofroxx, catalog number: 1125KG001)
9. CAPS (Sigma-Aldrich, catalog number: C2632)
10. Hydrochloric acid (HCl) (Honeywell, catalog number: 30721)
11. Sodium hydroxide (NaOH) (Honeywell, catalog number: 30620)
Solutions
1. Azo-Xylan substrate (see Recipes)
2. Citrate buffer (see Recipes)
3. Sodium phosphate buffer pH 6.0 (see Recipes)
4. Sodium phosphate buffer pH 7.0 (see Recipes)
5. Sodium phosphate buffer pH 8.0 (see Recipes)
6. Potassium phosphate buffer (see Recipes)
7. Tris-HCl buffer (see Recipes)
8. CAPS buffer (see Recipes)
9. Precipitant solution (see Recipes)
Recipes
1. Azo-Xylan substrate (100 mL)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Azo-Xylan powder | 1% w/v | 1 g |
| Distilled water (dH2O) | n/a | to 100 mL, see note |
| Total | 1% w/v | 100 mL |
Note: The Azo-Xylan powder should be added to 80 mL of boiling distilled water under vigorous stirring on a hot plate stirrer. Turn off the heat and continue stirring until the suspension has mixed completely. Add water to 100 mL.
2. Citrate buffer (100 mL)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Citric acid | 2.63 M | 9.60 g |
| HCl (6 N) | n/a | to pH 3.0, 4.0, or 5.0 |
| dH2O | n/a | to 100 mL, see note |
| Total | n/a | 100 mL |
Note: Dissolve the citric acid in 80 mL of dH2O. Adjust the pH to the desired value. Add water to 100 mL.
3. Sodium phosphate buffer pH 6.0 (100 mL)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| NaH2PO4 solution (1 M) | 438 mM | 43.80 mL |
| Na2HPO4 solution (1 M) | 62 mM | 6.20 mL |
| dH2O | n/a | to 100 mL |
| Total | n/a | 100 mL |
4. Sodium phosphate buffer pH 7.0 (100 mL)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| NaH2PO4 solution (1 M) | 195 mM | 19.50 mL |
| Na2HPO4 solution (1 M) | 305 mM | 30.50 mL |
| dH2O | n/a | to 100 mL |
| Total | n/a | 100 mL |
5. Sodium phosphate buffer pH 8.0 (100 mL)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| NaH2PO4 solution (1 M) | 34 mM | 3.40 mL |
| Na2HPO4 solution (1 M) | 466 mM | 46.60 mL |
| dH2O | n/a | to 100 mL |
| Total | n/a | 100 mL |
6. Potassium phosphate buffer (100 mL)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| KH2PO4 | 0.50 M | 6.80 g |
| HCl (6 N) | n/a | to pH 6.0 or 7.0 |
| dH2O | n/a | to 100 mL, see note |
| Total | n/a | 100 mL |
Note: Dissolve KH2PO4 in 80 mL of dH2O. Adjust the pH to the desired value. Add water to 100 mL.
7. Tris-HCl buffer (100 mL)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Tris-base powder | 0.50 M | 6.06 g |
| HCl (6 N) | n/a | to pH 7.0, 8.0 or 9.0 |
| dH2O | n/a | to 100 mL, see note |
| Total | n/a | 100 mL |
Note: Dissolve the Tris-base powder in 80 mL of dH2O. Adjust the pH to the desired value. Add water to 100 mL.
8. CAPS buffer (100 mL)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| CAPS powder | 0.50 M | 11.06 g |
| NaOH (5 M) | n/a | to pH 10.0, 11.0 |
| dH2O | n/a | to 100 mL, see note 5 |
| Total | n/a | 100 mL |
Note: Dissolve the CAPS powder in 80 mL of dH2O. Adjust the pH to the desired value. Add water to 100 mL.
9. Precipitant solution (100 mL)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Ethanol 95% | 95% v/v | 100 mL |
| Total | 95% v/v | 100 mL |
Laboratory supplies
1. 1.5 mL plastic tubes (Sarstedt, catalog number: 72.690.001)
2. 200 μL pipette tips (Manufacturer, catalog number: 70.3030.020)
3. 1000 μL pipette tips (Manufacturer, catalog number: 70.3050)
4. 96-well plates (Sarstedt, catalog number: 83.3924.500)
Equipment
1. LLG-uniTHERMIX Pro thermo shaker (Lab Logistics Group GmbH, catalog number: 6.263 484)
2. Centrifuge (Eppendorf, model: 5430 R)
3. BioTek Synergy Neo2 hybrid multi-mode reader (Agilent Technologies, model: BTNEO2)
Software and datasets
1. Gen5TM Microplate Reader and Imager Software (BioTek Instruments, Inc., version 3.10)
2. GraphPad Prism 9
3. Excel
Procedure
文章信息
稿件历史记录
提交日期: Jan 17, 2025
接收日期: Mar 18, 2025
在线发布日期: Apr 3, 2025
出版日期: Apr 20, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/).
如何引用
Bombardi, L., Coltro, A. and Fusco, S. (2025). Endo-1,4-β-D-xylanase Assay Using Azo-Xylan and Variants Thereof. Bio-protocol 15(8): e5283. DOI: 10.21769/BioProtoc.5283.
分类
生物化学 > 蛋白质 > 活性
微生物学 > 微生物生物化学 > 蛋白质 > 活性
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link