- EN - English
- CN - 中文
An Integrated Workflow for Three-Dimensional Visualization of Human Skeletal Muscle Stem Cell Nuclei
人骨骼肌干细胞细胞核的三维可视化集成流程
发布: 2025年04月20日第15卷第8期 DOI: 10.21769/BioProtoc.5281 浏览次数: 2496
评审: Xiaokang WuTien Anh NgoGiovanna Piovani
Abstract
Skeletal muscle–specific stem cells are responsible for regenerating damaged muscle tissue following strenuous physical activity. These muscle stem cells, also known as satellite cells (SCs), can activate, proliferate, and differentiate to form new skeletal muscle cells. SCs can be identified and visualized utilizing optical and electron microscopy techniques. However, studies identifying SCs using fluorescent imaging techniques vary significantly within their methodology and lack fundamental aspects of the guidelines for rigor and reproducibility that must be included within immunohistochemical studies. Therefore, a standardized method for identifying human skeletal muscle stem cells is warranted, which will improve the reproducibility of future studies investigating satellite activity. Additionally, although it has been suggested that SC shape can change after exercise, there are currently no methods for examining SC morphology. Thus, we present an integrated workflow for three-dimensional visualization of satellite cell nuclei, validated by the spatial context of the fluorescent labeling and multichannel signal overlap. Our protocol includes, from start to finish, post-biopsy extraction and embedding, tissue sectioning, immunofluorescence, imaging steps and acquisition, and three-dimensional data post-processing. Because of the depth volume generated from the confocal microscope z-stacks, this will allow future studies to investigate the morphology of SC nuclei and their activity, instead of traditionally observing them in two-dimensional space (x, y).
Key features
• Detailed instructions on post-biopsy extraction and embedding, tissue sectioning, immunofluorescence, imaging steps and acquisition, and three-dimensional data post-processing of muscle stem cells.
• Builds upon the validated method developed by Feng et al. [1], which was optimized for mouse tissue and fills critical gaps in existing literature.
• Allows qualitative and quantitative morphological assessment of muscle stem cell nuclei in three-dimensional space.
Keywords: Satellite cells (卫星细胞)Graphical overview
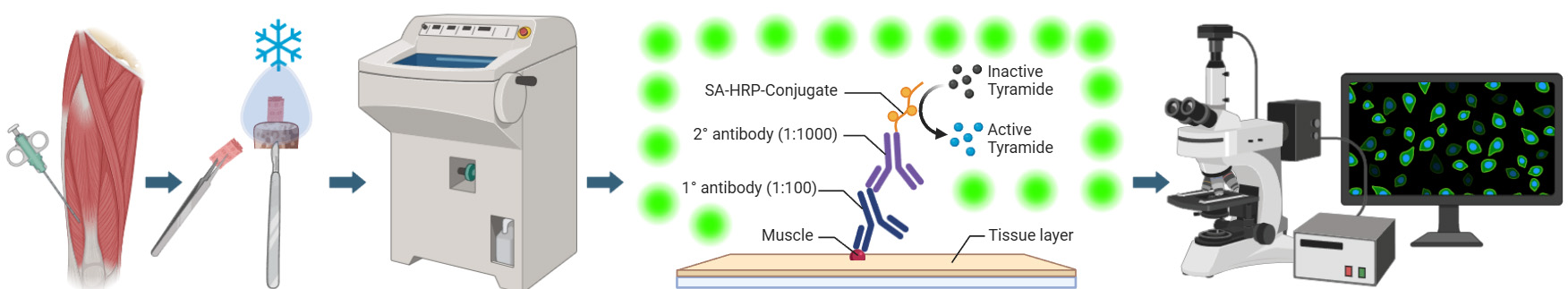
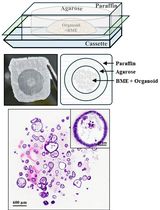
Graphical overview of integrated workflow for three-dimensional visualization of human skeletal muscle stem cells. After the percutaneous muscle biopsy, cut ~25–100 mg of the sample and arrange it according to the desired orientation → Mount sample for sectioning, embed in mounting medium, and freeze in liquid nitrogen-cooled isopentane → Using a cryostat, generate tissue cross-sections in an alternating collection method of 20 μm intervals and place on subbed glass slides → Fix and block sections before incubating in a cocktail of primary antibodies specific for satellite cell nuclei (anti-Pax7) and muscle membrane (anti-laminin) overnight. The following day, incubate sections in the appropriate secondary antibodies (Pax7: goat anti-mouse IgG1 biotin conjugated; laminin: goat anti-rabbit Alexa Fluor 488), apply signal amplification using streptavidin-horseradish peroxidase and tyramide 594 conjugate before counterstaining with DAPI, add mounting media, and coverslip → Using a confocal microscope, search for Pax7 signal, confirm overlap with DAPI adjacent to laminin labeling, apply appropriate laser channels, determine z-stack size, and acquire images in high-pixel-resolution format → For image post-processing, in the software’s three-dimensional viewer, modify individual channel histograms to optimize image quality and save as a TIFF.
Background
Intense physical activity can induce acute skeletal muscle damage that can persist for multiple days and even up to a few weeks [2]. In individuals with muscle-specific diseases such as sarcopenia, muscular dystrophy, or cachexia, this damage can be exacerbated due to impaired muscle regeneration mechanisms, often leading to progressive muscle wasting and functional decline [3]. Muscle stem cells, also referred to as satellite cells (SCs), are responsible for the repair of those injured fibers. Once damage occurs, SCs can activate, proliferate, and differentiate to assist in the recovery process. In addition to the differentiation process, SCs can self-renew, allowing for the quiescent SC pool to maintain its abundance. The path of the SC (i.e., differentiation, self-renewal, etc.) is contingent on myogenic transcription factors, which dictate the fate of the SC (Bellamy et al. [4]; Figure 1). These myogenic biomarkers can be visualized for identifying SC activity through fluorescent imaging. It is essential to study and understand these cells due to their crucial role following exercise and on skeletal muscle health and longevity; it has also been shown that SC exhaustion is linked to sarcopenia, which significantly increases the risk of mortality [5,6].
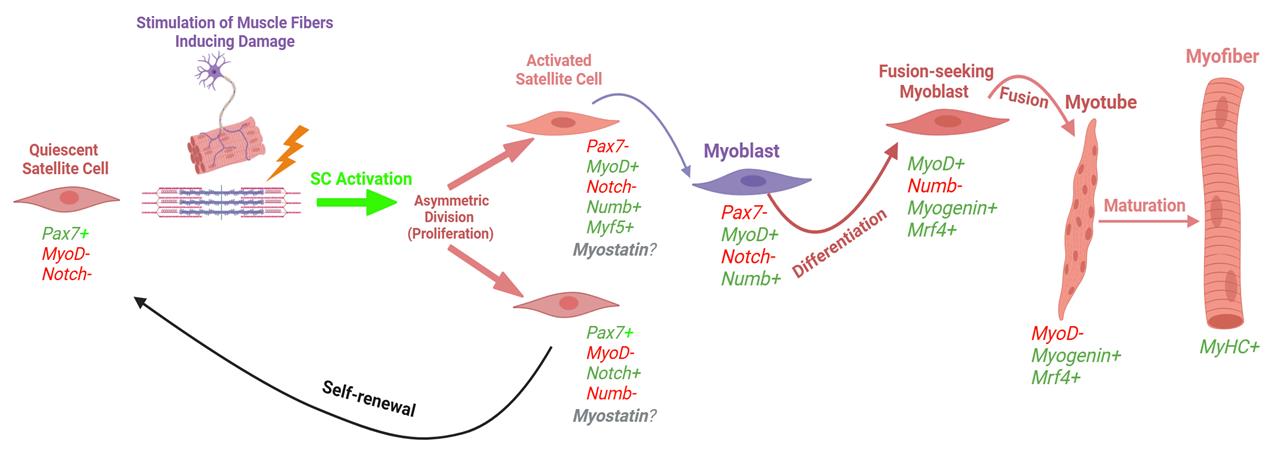
Figure 1. Myogenic lifespan of a satellite cell following exercise-induced muscle damage (adapted, modified, and used with permission from Vlasman [7]). Schematic representation of the two routes that satellite cells can take upon activation. Activated satellite cells can proliferate and then either commit to differentiation (expressing myogenin, MyoD, and MRF4) or self-renew back into a quiescent state (only expressing Pax7).
A fundamental area of skeletal muscle biology research has been identifying and monitoring the activity of satellite cells. One of the techniques for investigating SCs is the application of indirect immunofluorescence. This technique involves using a specific primary antibody that can bind to an epitope on the SC; then, with the help of a fluorophore-conjugated secondary antibody bound to the primary antibody, SCs can be visualized with a fluorescent microscope. For example, Pax7, a transcription factor highly expressed within the nuclei of SCs, has been suggested to be one of the most utilized primary antibodies for identifying SCs [1]. However, this is challenging, as SC nuclei comprise only 2%–7% of the total muscle-associated nuclei (e.g., myonuclei) in healthy adults and even less in elderly populations [8], making them hard to locate in small tissue samples.
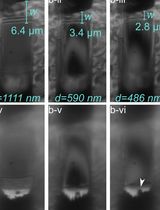
This protocol was designed to address key methodological gaps in previous literature. The SC identification protocols within the literature are missing fundamental aspects of the guidelines for rigor and reproducibility that must be included within immunohistochemical studies [9]. For example, there is a high degree of variability in the reagents used (e.g., antibodies, buffers, use of signal amplification products, or dilutions), experimental instructions, and published images. Also, these methodologies lack essential components within the experimental procedures including positive and negative controls, imaging instructions, and quantification of images. This reduces the potential for other laboratories to generate reproducible data. Additionally, to our knowledge, all studies investigating human SCs have sectioned the muscle tissue between 5 and 12 μm, which is problematic because SC length can reach up to 15 μm [10–12]. Specifically, cutting relatively thin sections can increase the possibility of error when analyzing SC quantity, due to the chance for duplication counts of the same cell on different sections. Therefore, we provide an optimized, detailed, and comprehensive protocol for visualizing human skeletal muscle SC nuclei in three-dimensional space, enabling the identification and morphological analysis of quiescent SC nuclei. This protocol was designed to address the key methodological gaps and has the potential to offer multiple advantages, including the ability to identify morphological changes in SC nuclei at rest, after exercise or nutritional interventions, and in healthy vs. diseased states.
Materials and reagents
Biological materials
1. Human skeletal muscle biopsy sample (male; 28 years old; 79 kg, 172 cm)
2. C2C12 (mouse myoblast) cell line (ATCC; CRL-1772) used as positive and negative controls
Reagents
1. Liquid nitrogen
2. Deionized water (diH2O)
3. Gum tragacanth (Sigma-Aldrich, catalog number: G1128)
4. 2-Methylbutane/isopentane 97.0+% (Thermo Fisher Scientific, catalog number: M0167500ML)
5. Paraformaldehyde (Sigma-Aldrich, catalog number: P6148-1KG)
6. Fisher optimal cutting temperature (OCT) compound (Thermo Fisher Scientific, catalog number: 23-730-571)
7. Antifade mounting media (VectaShield, Vector Laboratories, catalog number: H-1000)
8. 10× Phosphate buffered saline solution (PBS) (Thermo Fisher Scientific, catalog number: 70-011-044)
9. Saponin (Sigma-Aldrich, catalog number: 84510)
10. Sucrose (Thermo Fisher Scientific, catalog number: S5)
11. Hanks’ balanced salt solution (HBSS) 10× (Thermo Fisher Scientific, catalog number: 14185-052)
12. Dulbecco’s modified Eagle’s medium, high glucose (4.5 g/L) + L-glutamine without sodium pyruvate (Corning, Life Sciences, catalog number: 10-017-CV)
13. GibcoTM fetal bovine serum (FBS), qualified (Thermo Fisher Scientific, catalog number: 26140079)
14. Penicillin-streptomycin (10,000 U/mL) (Thermo Fisher Scientific, catalog number: 151440122)
15. Normal goat serum (Jackson ImmunoResearch, catalog number: 005-001-121)
16. 30% hydrogen peroxide (H2O2) (Thermo Fisher Scientific, catalog number: H325-100)
17. 2.5% normal horse serum (NHS) (Jackson ImmunoResearch, catalog number: 008-000-001)
18. Acetone (fixative solution) (Thermo Fisher Scientific, catalog number: A16P-4)
19. 4',6-diamidino2-phenylindole (DAPI) (Thermo Fisher Scientific, catalog number: D1306)
20. Type F immersion liquid (Leica Microsystems, catalog number: 11513859)
21. Gelatin (Electron Microscopy Sciences, catalog number: 16562)
22. Chromium potassium sulfate (Sigma-Aldrich, catalog number: 243361)
23. Immunohistochemistry reagents (Table 1)
Table 1. Immunohistochemistry antibodies and reagent information
| Antibody/reagent | Company | Catalog number | Reactivity | Host/isotype | Dilution/diluent | Storage |
| Pax7 (concentrate) monoclonal primary antibody Caution: We could not optimize this protocol with the supernatant (dilutions 1:10, 1:50, and 1:100) or a different Pax7 primary antibody (Rabbit anti-Pax7 polyclonal IgG; Thermo Fisher Scientific, cat no.: PA1-117) | Developmental Studies Hybridoma Bank | Pax7 | Human | Mouse/IgG1 | (1:100)/2.5% NHS | -20 °C |
| Biotinylated-SP goat anti-mouse IgG1 secondary antibody | Jackson ImmunoResearch | 115-065-205 | Mouse IgG1 | Goat/IgG1 | (1:1,000)/2.5% NHS | -20 °C |
| Laminin polyclonal primary antibody | Sigma-Aldrich | L9393 | Human | Rabbit/IgG | (1:200)/2.5% NHS | -20 °C |
| Alexa Fluor 488 goat anti-rabbit IgG secondary antibody | Thermo Fisher Scientific | A-11034 | Rabbit | Goat/IgG | (1:200)/PBS | 4 °C |
| Alexa Fluor 594 tyramide | Thermo Fisher Scientific | B40957 | HRP | N/A | (1:200)/PBS | 4 °C |
| Streptavidin horseradish peroxidase (SA-HRP) | Thermo Fisher Scientific | S911 | Biotin | N/A | (1:500)/PBS | -20 °C |
| Alexa Fluor 647 goat anti-mouse secondary antibody Caution: This secondary antibody was used with the supernatant primary antibody, which we could not optimize for this protocol | Thermo Fisher Scientific | A21236 | Mouse | Goat/IgG | (1:200)/1× HBSS + 0.1% sucrose + 1% saponin | 4 °C |
Note: Prepare on the day of the experiment.
Solutions
1. 1× Phosphate buffered saline (PBS) (see Recipes)
2. Subbing solution for glass slides (see Recipes)
3. 2.5% Normal horse serum (blocking buffer) (see Recipes)
4. 3% hydrogen peroxide (see Recipes)
5. Ice-cold acetone fixative solution (see Recipes)
6. 10 mM 4',6-diamidino2-phenylindole (DAPI) (see Recipes)
Recipes
1. 1× Phosphate buffered saline (PBS) (100 mL)
| Reagent | Quantity/Volume |
|---|---|
| PBS 10× | 10 mL |
| diH2O | 900 mL |
Prepare the day before. Store at 4 °C.
2. Subbing solution for glass slides (1 L)
| Reagent | Quantity/Volume |
|---|---|
| Gelatin | 1 g |
| Chromium potassium sulfate | 0.1 g |
| Hot distilled H2O | 1 L |
Mix well and let it cool before adding chromium potassium sulfate. Submerge slides and place them vertically on a dry paper towel. Stock can be stored at 4 °C; aliquot working volumes in a coupling jar.
3. 2.5% normal horse serum (blocking buffer) (3 mL)
| Reagent | Quantity/Volume |
|---|---|
| Normal horse serum | 75 μL |
| 1× PBS | 2,925 μL |
Prepare the day before. Store at 4 °C.
4. 3% hydrogen peroxide (1 mL)
| Reagent | Quantity/Volume |
|---|---|
| 30% H2O2 | 30 μL |
| 1× PBS | 970 μL |
Prepare the day before. Store at 4 °C.
5. Ice-cold acetone fixative solution (1 mL)
| Reagent | Quantity/Volume |
|---|---|
| Acetone | 1 mL |
Prepare on ice the day before. Store at -20 °C.
6. 10 mM 4',6-diamidino2-phenylindole (DAPI) (50 mL)
| Reagent | Quantity/Volume |
|---|---|
| 14.3 mM DAPI | 35 μL |
| diH2O | 49.955 μL |
Prepare the day before. Reconstitute with 2 mL of diH2O. Store at 4 °C; if protected from light, it is usable long-term.
Laboratory supplies
1. Petri dishes 100 mm Nunc (Thermo Fisher Scientific, catalog number: 263991)
2. Reynold wrap aluminum foil (Grainger, catalog number: 6CHG6)
3. Superfrost Plus slides (Thermo Fisher Scientific, catalog number: 12-550-15)
4. 22 × 50 mm No. 1.5 (0.16–0.19 mm) thick coverslip glass (Ted Pella, catalog number: 260154)
5. Ibidi μ-slide 8-well glass bottom (Ibidi, catalog number: 80827)
6. Kimwipes (KCWW, Kimberly-Clark, catalog number: 34120)
7. Gloves (VWR, catalog number: 82026-424)
Equipment
1. Bergström needle (5 mm) composed of the outer cannula, inner trocar, and plunger (Pelomi Medical, Albertslund, Denmark, catalog number: 1226-005) with associated components: 200 μL pipette tip with ~15–18 mm cutoff, 30 cm extension tubing, and disposable 140 mL Monoject bulk syringe (Cardinal Health, catalog number: 8881114055)
2. Lab-grade stopper cork (size 00) for muscle mount (Thermo Fisher Scientific, catalog number: 07-782G)
3. Swann-Morton scalpel with blade size 11, sterile (Thermo Fisher Scientific, catalog number: 50-192-8439)
4. Styrofoam cryo dewar (for liquid nitrogen) (Agar Scientific, catalog number: AG81760-130)
5. Cryostat microtome (Leica Microsystems, model: CM1800)
6. HP35 ultra-microtome disposable blades at 34°/75 mm (Thermo Fisher Scientific, catalog number: 31-537-35)
Note: We recommend that slides be coated with subbing solution before collecting samples.
7. Humidifying slide chamber (6-slide clear staining tray with clear lid) (Sigma-Aldrich, catalog number: H6644-1EA)
8. SpectrafugeTM 16M brushless laboratory microcentrifuge (Labnet, catalog number: C0160)
9. 0.5–10 μL mechanical pipette (Eppendorf, catalog number: 3123000020)
10. 20–200 μL mechanical pipette (Eppendorf, catalog number: 3123000055)
11. 100–1,000 μL mechanical pipette (Eppendorf, catalog number: 3123000063)
12. 500 μL plastic microcentrifuge tubes (Thermo Fisher Scientific, catalog number: AM12350)
13. 500 mL Pyrex round, wide-mouth media storage bottle with GLS 80 screw cap (Corning Life Sciences, catalog number: 1397-500)
14. Camel hair paint brush, fine tip (size 0.5–3) for sectioning (Grainger, catalog number: 39AL12)
15. Forceps, fine tip (Thermo Fisher Scientific, catalog number: 22-327379)
16. Clear nail polish for sealing slides (Thermo Fisher Scientific, catalog number: 50-949-071)
17. Rounded flat-end scoop spatula (Grainger, catalog number: 56HV79)
18. 4 °C refrigerator
19. Epifluorescent inverted microscope (Olympus, model: IX-81)
20. Laser scanning confocal upright microscope (Leica Microsystems, model: DM6-Q)
Software and datasets
1. LAS X digital imaging software version 3.5.7.23225 (Leica Biosystems)
2. BioRender (https://www.biorender.com/). The following figures were created using BioRender: Graphical overview; Figure 1)
Procedure
文章信息
稿件历史记录
提交日期: Dec 11, 2024
接收日期: Mar 12, 2025
在线发布日期: Mar 31, 2025
出版日期: Apr 20, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/).
如何引用
Pearson, J. R., Martinez-Rivera, N., Torres-Vasquez, I., Gallagher, P. M. and Rosa-Molinar, E. (2025). An Integrated Workflow for Three-Dimensional Visualization of Human Skeletal Muscle Stem Cell Nuclei. Bio-protocol 15(8): e5281. DOI: 10.21769/BioProtoc.5281.
分类
干细胞 > 成体干细胞 > 肌肉干细胞
细胞生物学 > 细胞成像 > 冷冻超薄切片
细胞生物学 > 组织分析 > 组织形态学
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link