- EN - English
- CN - 中文
In Vitro Bone Marrow–Derived Dendritic Cells (BMDC) Generation for Antigen Presentation Assay
体外诱导骨髓来源树突状细胞(BMDC)用于抗原呈递实验
(*contributed equally to this work) 发布: 2025年04月20日第15卷第8期 DOI: 10.21769/BioProtoc.5278 浏览次数: 4316
评审: Andrea GramaticaMartin V KolevRohini Ravindran Nair
Abstract
Dendritic cells (DC) are sentinel cells of the immune system that process and present antigens to activate T cells, thus serving to bridge the innate and adaptive immune systems. DCs are particularly efficient at cross-presentation whereby exogenously acquired antigens are processed and presented in context with MHCI molecules to activate CD8+ T cells. Assaying antigen presentation by DCs is a critical parameter in assessing immune functionality. However, the low abundance of bona fide DCs within the lymphoid compartments limits the utility of such assays. An alternative approach employing the culturing of bone marrow cells in the presence of factors needed for DC lineage commitment can result in the differentiation of bone marrow dendritic cells (BMDCs). This protocol details the process of in vitro generation of BMDCs and demonstrates their subsequent utility in antigen presentation assays. The protocol described can be adapted to various conditions and antigens.
Key features
• BMDCs can serve as surrogate antigen-presenting cells (APCs) for assessing in vitro and in vivo antigen presentation.
• Co-culture of antigen-stimulated BMDCs with CFSE-labeled T cells can help quantify the responsiveness of both the antigen presenters and responders.
• In vivo analysis of antigen presentation by BMDCs can be assessed using an adoptive transfer approach.
• CFSE labeling can help track in vivo the fate of adoptively transferred BMDCs as well as T cells.
Keywords: BMDCs (骨髓来源树突状细胞(BMDC))Graphical overview
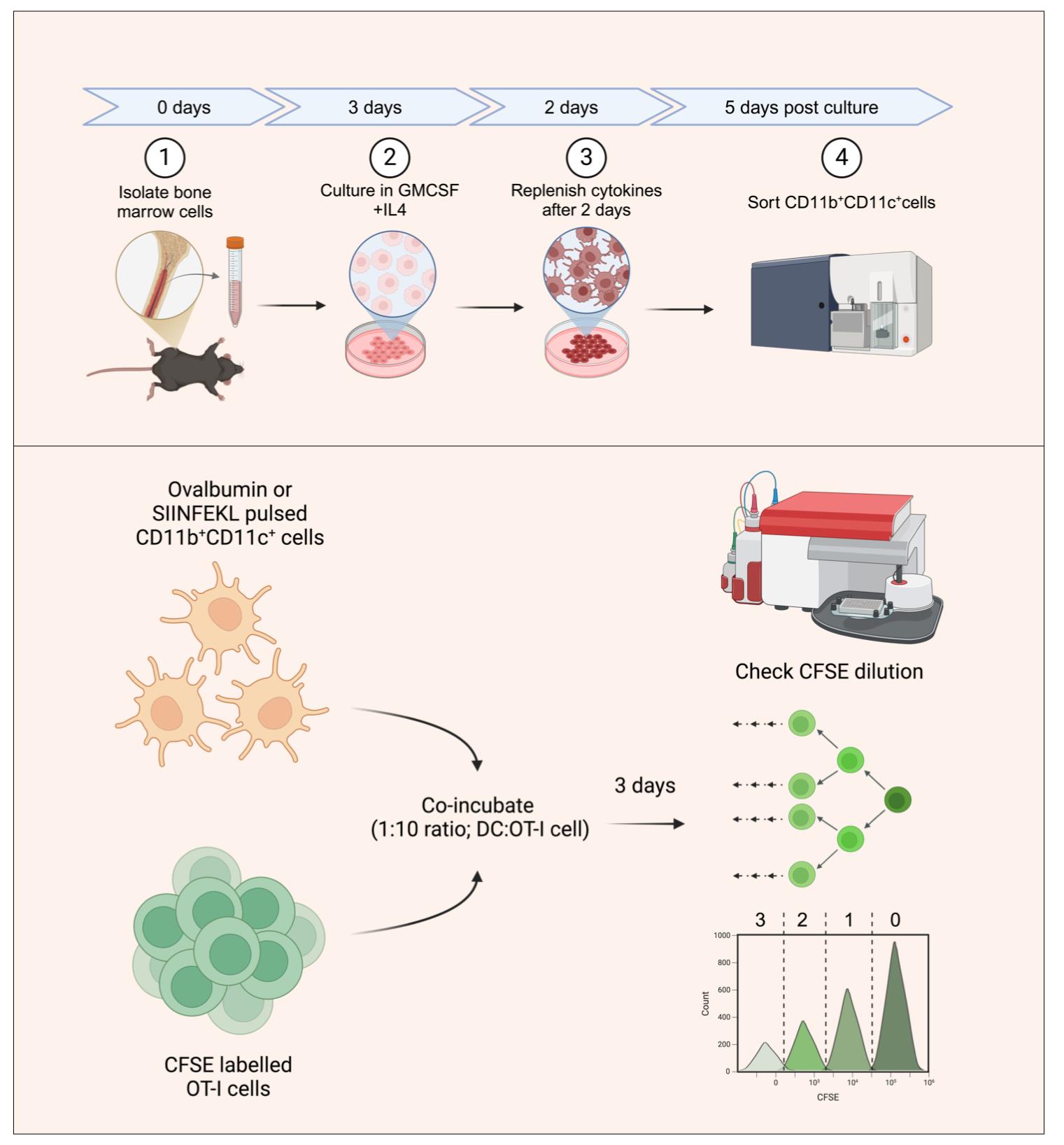
Generating bone marrow dendritic cells (BMDCs) for in vitro antigen presentation assays. The bone marrow precursors are cultured in the presence of interleukin 4 (IL-4) and granulocyte-macrophage colony-stimulating factor (GM-CSF) to generate DCs. BMDCs are then sorted as double-positive cells based on CD11b and CD11c staining. The sorted BMDCs are then pulsed with SIINFEKL peptide or ovalbumin protein and subsequently co-incubated with carboxyfluorescein succinimidyl ester (CFSE)-labeled OT-I T-cell receptor (TCR) transgenic cells with SIINFEKL-H-2Kb reactivity. The antigen presentation potential of the generated BMDCs is assayed by quantifying the frequencies of CFSE-diluting OT1 cells, with each peak representing a group of cells having undergone a different number of divisions.
Background
An important breakthrough in immunology research was the development of in vitro functionality assays. While developing the mixed lymphocyte reaction assay, Mishell and Dutton made the unexpected finding that the lymphocytes by themselves were unable to produce an immune response and required glass-adhering accessory antigen-presenting cells (APCs) [1]. Subsequently, the work of Steinman and Cohn [2,3] established morphologically distinct accessory cell types, which they named dendritic cells (from the Greek dendreon meaning tree, owing to their tree-like projections). In 1978, Steinman and Nussenzweig developed the first antigen-presentation assay and showed that DCs present antigens to T cells to initiate immunity [4]. However, due to the extremely laborious procedure of purifying these cells, studies of DCs remained restricted to the laboratory of Steinman and his colleagues. Dendritic cell biology witnessed significant advances in the 1990s when Sallusto and Lanzavecchia developed an assay to differentiate DCs from blood monocytes by culturing them in the presence of granulocyte-macrophage colony-stimulating factor (GM-CSF) and interleukin (IL)-4 [5].
It is now well known that DCs are the main APCs involved in the recruitment of virus-specific CD8+ T cells during an infection. Peptides derived from the processing of endogenous proteins or those acquired exogenously by the DCs are presented on the cell surface via the class I MHC molecules to specific CD8+ T cells [6,7]. The peptide-MHC-I complexes engage the specific TCR-expressing CD8+ T cells (cytotoxic T lymphocytes, CTL), thus providing the first of three signals required for T-cell recruitment. The co-stimulatory receptor (B7.1/7.2) and ligand pair (CD28) are then engaged, which further enforces the T-cell activation program, which is fine-tuned by the generated cytokine milieu, also considered as signal II and III, respectively. The generation of an optimal CD8+ T-cell response is therefore contingent upon the proper functioning of multiple cellular processes within the DCs. These include 1) efficient uptake of exogenous antigens by the DCs, 2) processing of the internalized proteins into immunogenic peptides, 3) transport of the peptide-MHC complexes to the cell surface, 4) optimal expression of co-stimulatory molecules, and 5) optimal production of cytokines involved in T-cell priming. These events can efficiently be captured within the DCs by assaying their antigen presentation capability.
Here, we describe the generation of DCs from bone marrow precursors and subsequent analysis aimed at measuring their ability to prime antigen-specific CD8+ T cells by performing in vitro antigen presentation assays. To this end, we use the model CD8+ T-cell epitope, SIINFEKL (derived from the chicken egg ovalbumin, Ova257-264), as well as the complete ovalbumin protein to pulse bone marrow dendritic cells (BMDCs). The pulsed BMDCs were then co-incubated with OT1 cells (transgenic T cells having TCRs specific for the SIINFEKL epitope). The proliferation of the OT1 cells in response to stimulation with the pulsed BMDCs is then assayed using CFSE dilution. As such, the protocol described here provides a primer for studies aimed at assessing DC functionality in diverse conditions of health, disease, and aging and can be adapted to different epitopes and T-cell pairs.
Materials and reagents
Biological materials
1. C57BL/6 mice (wild-type B6 mice, Jackson Laboratory, strain# 000664)
2. C57BL/6-Tg (TcraTcrb)1100Mjb/J (OT1 TCR transgenic mice, Jackson Laboratory, strain# 003831)
Reagents
1. RPMI (Gibco, catalog number: 31800-022)
2. Fetal bovine serum (FBS) (Gibco, catalog number: 26140-079)
3. Penicillin-streptomycin (Gibco, catalog number: 10378-016)
4. CFSE (Thermo Fisher Scientific, Invitrogen, catalog number: C34570)
5. Trypan blue 0.4% solution in Dulbecco’s phosphate buffered saline (HIMEDIA, catalog number: TCL046)
6. Ovalbumin protein (SERVA, catalog number: 11842.01)
7. SIINFEKL peptide (GL Biochem, catalog number: 181660)
8. Murine GM-CSF (Thermo Fisher Scientific, PeproTech, catalog number: 315-03-20UG, product format: 5 ng/mL in SFRPMI)
9. Murine IL4 (Thermo Fisher Scientific, PeproTech, catalog number: 214-14-20UG, product format: 5 ng/mL in SFRPMI)
10. Anti-Mouse CD11b (BioLegend, catalog number: 101205)
11. Anti-Mouse CD11c (BioLegend, catalog number: 117317)
12. Anti-Mouse CD8a (BioLegend, catalog number: 100733)
13. Mouse CD8 T-Cell Isolation kit (BioLegend, catalog number: 480008)
14. Sodium chloride (MERCK, EMPARTA, CAS: 7647-14-5)
15. Potassium chloride (HIMEDIA, CAS: 7447-40-7)
16. Potassium dihydrogen phosphate (SERVA, catalog number: 7778-77-0)
17. Disodium hydrogen phosphate (HIMEDIA, CAS: 7558-79-4)
18. Ammonium chloride (MERCK, CAS: 12125-02-9)
19. Sodium bicarbonate (SERVA, CAS: 144-55-8)
20. EDTA (MERCK, catalog number: 1.93312.1021)
21. Dimethyl sulfoxide (DMSO) (Thermo Fisher Scientific, catalog number: 85190)
Solutions
1. FACS buffer (see Recipes)
2. 1× phosphate-buffered saline (PBS) (see Recipes)
3. RBC lysis buffer (see Recipes)
4. RPMI (see Recipes)
5. CFSE stock solution (see Recipes)
6. CFSE working solution (see Recipes)
7. SIINFEKL peptide stock solution (see Recipes)
8. Ovalbumin stock solution (see Recipes)
Recipes
1. FACS buffer
| Reagent | Final concentration | Quantity or Final Volume |
|---|---|---|
| 1× PBS | 100 mL | 100 mL |
| Fetal bovine serum | 2% | 2 mL |
Prepare fresh each time before proceeding with the experiment.
2. Phosphate buffered saline (PBS) (pH 7.4, for 1,000 mL)
| Reagent | Final concentration | Quantity |
|---|---|---|
| Sodium chloride | 137 mM | 8 g |
| Potassium chloride | 2.7 mM | 0.201 g |
| Potassium dihydrogen phosphate | 2 mM | 0.260 g |
| Disodium hydrogen phosphate | 10 mM | 1.42 g |
Store the PBS at 4 °C and warm it at room temperature before use.
3. RBC lysis buffer (pH 7.3, for 750 mL)
| Reagent | Final concentration | Quantity |
|---|---|---|
| Ammonium chloride | 155 mM | 6.218 g |
| Sodium bicarbonate | 12 mM | 0.756 g |
| EDTA | 0.1 mM | 27.918 mg |
Store the RBC lysis buffer solution at 4 °C.
4. RPMI (pH 7.3, for 500 mL)
| Reagent | Final concentration | Quantity |
|---|---|---|
| Serum-free RPMI | 1× | 445 mL |
| Penicillin-streptomycin | 1× | 5 mL |
| FBS | 1× | 50 mL |
Store the complete medium at 4 °C.
5. CFSE stock solution (18 µL)
| Reagent | Stock concentration | Quantity |
|---|---|---|
| CFSE dye | 5 mM | NA |
| DMSO | 1× | 18 μL |
Store the resuspended stock at -20 °C.
6. CFSE working solution (500 μL)
| Reagent | Final concentration | Quantity |
|---|---|---|
| CFSE stock solution | 5 mM | 0.5 μL |
| Serum-free RPMI | 1× | 500 μL |
Prepare fresh each time before proceeding with the experiment.
Note: The above working solution is shown for labeling ~10 million cells resuspended in 500 μL of serum-free RPMI.
7. SIINFEKL peptide stock solution (5 mL)
| Reagent | Final concentration | Quantity |
|---|---|---|
| SIINFEKL peptide (lyophilized) | 10 mg/mL | 50 mg |
| DMSO | - | 5 mL |
Aliquot and store the peptide at -80 °C.
8. Ovalbumin stock solution (5 mL)
| Reagent | Final concentration | Quantity |
|---|---|---|
| Ovalbumin protein (lyophilized) | 20 mg/mL | 100 mg |
| RPMI | - | 5 mL |
Aliquot and store the stock solution at -80 °C.
Laboratory supplies
1. 100 mm Petri dish (Cole-Parmer, catalog number: 15971-43)
2. Round-bottom 96-well cell culture plate (Corning, catalog number: 3799)
3. 500 mL vacuum filter/storage bottle system 0.22 μm pore 33.2 cm2 PES membrane (Corning, catalog number: 431097)
4. 50 mL centrifuge tubes (Tarsons, catalog number: 546042)
5. 15 mL centrifuge tubes (Tarsons, catalog number: 546022)
6. 1.5 mL microcentrifuge tubes (Tarsons, catalog number: 500010)
7. 1,000 μL tips (Tarsons, catalog number: 521020)
8. 200 μL tips (Tarsons, catalog number: 521014)
9. 10 μL tips (Tarsons, catalog number: 521000)
10. Cell strainer, 70 μm (Cole-Parmer, catalog number: 04396-01)
11. Serological pipettes (Eppendorf, catalog number: 0030127730)
12. Cell scraper (Cole-Parmer, catalog number: 04396-53)
Equipment
1. Biosafety cabinet (Labconco, model: Logic Class II, Type A2 Biosafety Cabinets)
2. CO2 incubator (Eppendorf, model: NB-170R)
3. Centrifuge (Eppendorf, model: AG 5810 R)
4. Inverted microscope (Olympus, model: IX53)
5. BD FACSAriaTM Fusion cell sorter (BD Biosciences)
6. BD Accuri C6 Flow cytometer (BD Biosciences)
7. Magnetic bars (Tarsons, catalog number: 4117)
8. Serological pipettes and micropipettes (Eppendorf Research Plus)
9. Cell counter (Marienfeld, hemocytometer 0640010)
10. Laboratory roller (Neuation, model: iRoll PR35)
Software and datasets
1. BD FACSDiva software (Version 8.0.2)
2. GraphPad Prism (Version 9)
3. FlowJo (Version X)
4. BioRender (https://www.biorender.com/). The following figures were created using BioRender: Graphical overview, https://BioRender.com/uo9ctw9.
Procedure
文章信息
稿件历史记录
提交日期: Jan 6, 2025
接收日期: Mar 13, 2025
在线发布日期: Mar 28, 2025
出版日期: Apr 20, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/).
如何引用
Singh, S., Tehseen, A., Iqbal, M. S. and Sehrawat, S. (2025). In Vitro Bone Marrow–Derived Dendritic Cells (BMDC) Generation for Antigen Presentation Assay. Bio-protocol 15(8): e5278. DOI: 10.21769/BioProtoc.5278.
分类
免疫学 > 免疫细胞功能 > 树突细胞
细胞生物学 > 细胞分离和培养 > 共培养
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link