- EN - English
- CN - 中文
High-Performance Liquid Chromatography Quantification of Glyphosate, Aminomethylphosphonic Acid, and Ascorbate in Culture Medium and Microalgal Cells
高效液相色谱法检测培养基及微藻细胞中草甘膦、氨基甲基膦酸和抗坏血酸的含量
发布: 2025年04月05日第15卷第7期 DOI: 10.21769/BioProtoc.5263 浏览次数: 1168
评审: Noelia ForesiPhilipp A.M. SchmidpeterWalmik Karbhari Gaikwad
Abstract
Glyphosate (GLY) is a widely used herbicide that can induce oxidative stress in microalgae and other non-target organisms. The quantification of GLY in surface water is a difficult task, especially in trace-level concentrations, due to its high polarity and susceptibility to biotic and abiotic degradation. Several analytical methods have been developed for GLY quantification. Most of them use high-performance liquid chromatography (HPLC) coupled with detection by mass spectrometry (MS) and include a derivatization step to decrease the polarity of the herbicide to improve detection. This protocol describes an adaptation of an existing protocol for the quantification of GLY and its metabolite aminomethylphosphonic acid (AMPA) in a water-based microalgae culture medium using ultra-high-pressure liquid chromatography (UHPLC) coupled with fluorescence detection (FLR). The principal advantage of this protocol compared with other analytical methods that employ HPLC–MS is its low cost and accessibility since it does not require an MS detector nor radioactively labeled analytical standards. Ascorbic acid (AH-) is one of the most important hydrosoluble non-enzymatic antioxidants in eukaryotic cells and plays a key role in many metabolic pathways of critical importance in plants and algae. In this protocol, we also describe an adaptation of a previously published protocol to quantify AH- in blood samples to be used in microalgal cells exposed to GLY and GLY-based herbicides. The sample preparation procedure for this last protocol is fast, easy, and does not require expensive equipment. It uses an HPLC system coupled with an electrochemical detector (EC) for AH- quantification but may be adapted to be used with a UV-Vis detector.
Key features
• These protocols employ HPLC or UHPLC. The end user should be proficient with this technique and be able to optimize chromatographic parameters as necessary.
• The GLY and AMPA quantification protocol, designed upon the method developed by Nedelkoska [1], expands its use for water-based cell culture media.
• The protocol used for AH-, designed upon the method developed by Kutnink [2], has been modified to be used in microalgae cells.
• The quantification of AH- employs HPLC coupled with an electrochemical detector but could be modified for its use with a UV-VIS detector.
Keywords: Glyphosate (草甘膦)Graphical overview
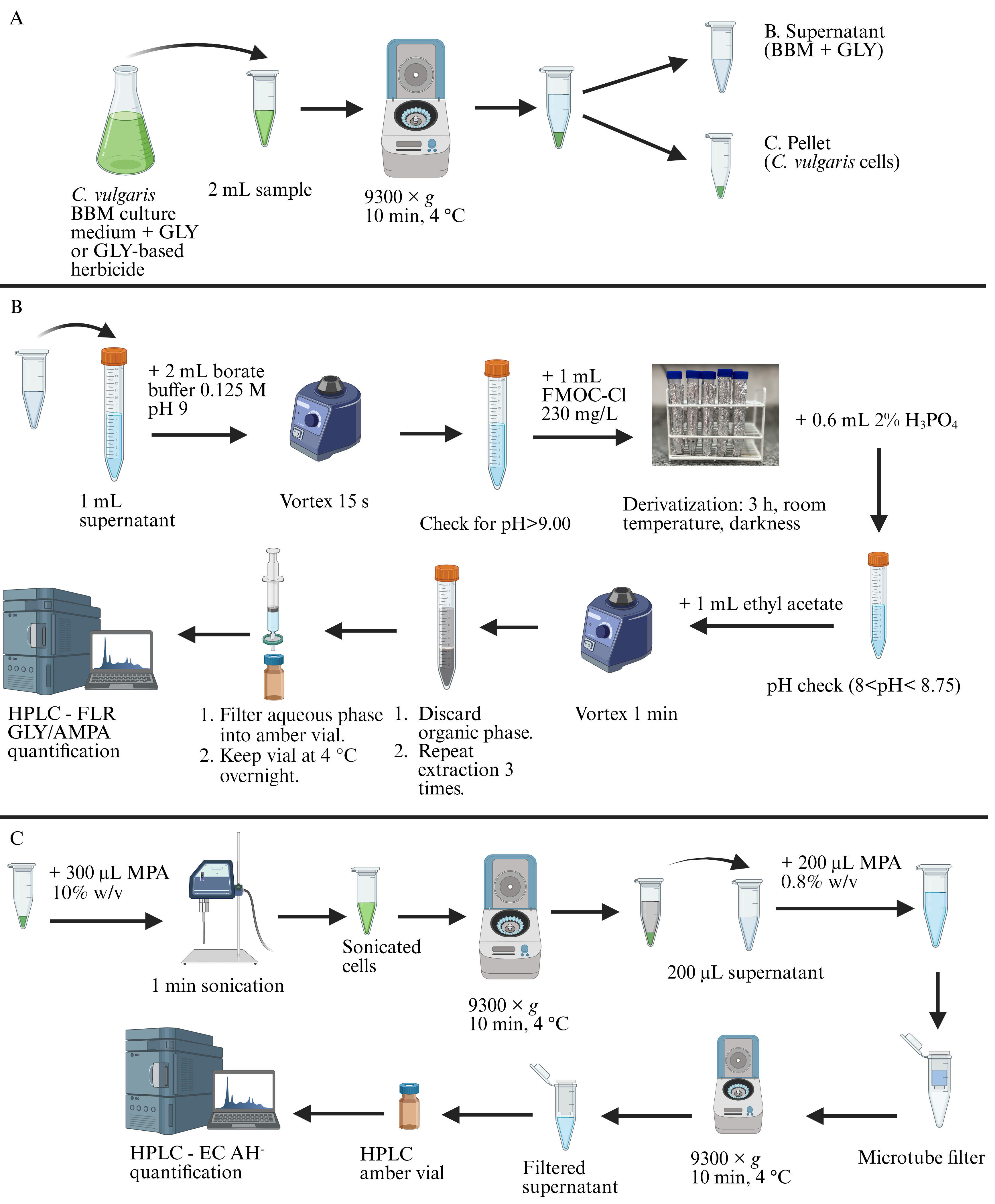
Glyphosate (GLY), aminomethylphosphonic acid (AMPA), and L-ascorbic acid (AH-) extraction and/or derivatization. (A) Cell harvest procedure. (B) Extraction and derivatization of GLY and AMPA in cell culture medium. (C) Extraction of AH- from microalgal cells. BBM: Bold’s basal medium. HPLC: High-performance liquid chromatography. FLR: Fluorescent detector. EC: Electrochemical detector. FMOC-Cl: 9-fluorenilmethyl chloroformate. MPA: Metaphosphoric acid.
Background
In this article, we describe two analytical protocols for the quantification of glyphosate (GLY) and ascorbic acid (AH-) in microalgal cultures exposed to an analytical standard or a GLY-based herbicide. The first protocol described here is based on a method originally published in 2004 [1] that has been adapted to quantify GLY and its metabolite aminomethylphosphonic acid (AMPA) in a water-based culture medium. Several modifications have been made to the original method to adapt it to the equipment available in our laboratory. The main differences are related to the type of chromatographic column and mobile phase used and the derivatization step. In our protocol, several key steps of the derivatization process were identified and optimized to improve efficiency, including carefully controlling the pH of the derivatization reaction and the order in which the reagents are added. The total run time of the analysis was also improved as compared to the original method using a UHPLC system. The use of a fluorescence (FLR) detector allows this protocol to have reduced costs as it is a relatively common detector found in most standard HPLC systems. This is also the key disadvantage of our protocol because FLR detection requires a clean sample to successfully quantify the derivatized analytes. However, this method is suitable for the analysis of both GLY and AMPA in drinking and superficial water samples and has been successfully implemented for the quantification of these analytes in water-based culture media.
The second protocol described here is used to quantify ascorbate ion (AH-) in microalgae cells. Different spectrophotometric, fluorescent, and chromatographic methods exist, which allow the determination of appreciable amounts of this antioxidant [3]. However, few methods allow direct and simultaneous detection of several chemical species. HPLC detection using different types of detection (electrochemical, fluorescent, etc.) establishes a rapid, sensitive, selective, and reproducible method for the detection of AH- [4]. Electrochemical detection (EC) is an attractive alternative method for the detection of electroactive species because of its inherent advantages of simplicity, ease of access to miniaturization, high sensitivity, and relatively low cost. EC typically works in amperometric or coulometric mode and can be coupled with HPLC to provide high sensitivity to electroactive species [5]. The protocol described here adapts one originally published in 1987 [2] to be used with microalgal cells. It is a very simple and fast method that does not require complex processing of samples. Its main advantage (and disadvantage) is the requirement of an electrochemical detector; however, it may be replaced by a UV-VIS detector, provided that samples are thoroughly cleaned up before injection in the chromatographic system, because the pigments in microalgal and plant cells interfere with UV-VIS detectors, affecting the detection and quantification levels of this method.
Materials and reagents
Biological materials
1. Chlorella vulgaris CPCC90 (UTCC90) (Canadian Phycological Culture Centre, catalog number: CPCC90)
Reagents
GLY and AMPA analysis
1. Glyphosate analytical standard (AccuStandard, catalog number: P-015NB, CAS: 1071-83-06)
2. Aminomethyl phosphonic acid (AMPA) analytical standard (Sigma-Aldrich, catalog number: 324817, CAS: 1066-51-9)
3. Potassium dihydrogen phosphate (KH2PO4) for analysis EMSURE® ISO (Supelco, catalog number: 1.04873, CAS: 7778-77-0)
4. Sodium tetraborate decahydrate (Na2B4O7·10H2O) for analysis ACS (Supelco, catalog number: 106308, CAS: 1303-96-4)
5. 9-Fluorenilmethyl chloroformate (FMOC-Cl), 99% (Sigma-Aldrich, catalog number: 23184, CAS: 28920-43-6)
6. Sodium hydroxide (NaOH), ACS reagent 97%, pellets (Sigma-Aldrich, catalog number: 221465, CAS: 1310-73-2)
7. Acetonitrile isocratic grade for liquid chromatography LiChrosolv® (Supelco, catalog number: 100029, CAS: 75-05-8)
8. Methanol isocratic grade for liquid chromatography LiChrosolv® (Supelco, catalog number: 106018, CAS: 67-56-1)
9. Ethyl acetate ACS grade (Merck, catalog number: 109623, CAS: 141-78-6)
10. Hydrochloric acid fuming (HCl), 37% for analysis ACS (Supelco, catalog number: 100317, CAS: 7647-01-0)
11. Orthophosphoric acid (H3PO4), 85% for analysis ACS (Supelco, catalog number: 100573, CAS: 7664-38-2)
12. Type 1 ultrapure water [6], resistivity 18 MΩ/cm at 25 °C)
AH- analysis
1. Metaphosphoric acid (MPA) ACS reagent, chips, 33.5%–36.5% (Sigma-Aldrich, catalog number: 239275, CAS: 37267-86-0)
2. Type 1 ultrapure water [6], resistivity 18 MΩ/cm at 25 °C
3. L-ascorbic acid, 99% (Sigma-Aldrich, catalog number: A92902, CAS: 50-81-7)
Solutions
For GLY and AMPA quantification (see Recipes)
1. GLY stock solution
2. GLY intermediate solution 1 (GLYI1)
3. GLY intermediate solution 2 (GLYI2)
4. GLY intermediate solution 3 (GLYI3)
5. AMPA stock solution
6. AMPA intermediate solution 1 (AMPAI1)
7. AMPA intermediate solution 2 (AMPAI2)
8. AMPA intermediate solution 3 (AMPAI3)
9. GLY calibration curve (GLY 1; GLY 2; GLY 3)
10. AMPA calibration curve (AMPA 1; AMPA 2; AMPA 3)
11. FMOC-Cl stock solution
12. FMOC-Cl working solution
13. Sodium tetraborate decahydrate (Na2B4O7·10H2O) buffer 0.125 M pH 9
14. 3 M HCl
15. KH2PO4 buffer solution 50 mM pH 5.5
16. NaOH 10% w/v
17. H3PO4 4% v/v
For AH- quantification (see Recipes)
1. MPA 10% w/v
2. MPA 0.8% w/v
3. L-ascorbic acid stock solution (1 mg/mL or 1 mM in MPA 10%)
4. L-ascorbic acid working solution (1 μg/mL or 10 μM in MPA 10%)
Recipes
For GLY and AMPA quantification
1. GLY stock solution (1 mg/mL) (10 mL)
Note: Stock solutions must be stored in plastic tubes, covered with aluminum foil.
| Reagent | Quantity | Final concentration | Shelf life/storage conditions |
|---|---|---|---|
| GLY analytical standard | 10 mg | 1 mg/mL | 6 months/4 °C |
| Ultrapure water | 10 mL | ||
| Total | 10 mL |
2. GLYI1 (1 mL)
Note: The intermediate solutions must be stored in plastic tubes, covered with aluminum foil.
| Reagent | Quantity | Final concentration | Shelf life/storage conditions |
|---|---|---|---|
| GLY stock solution | 10 μL | 10 μg/mL | 48 h/4 °C |
| Ultrapure water | 990 μL | ||
| Total | 1,000 μL |
3. GLYI2 (1 mL)
Note: The intermediate solutions must be stored in plastic tubes, covered with aluminum foil.
| Reagent | Quantity | Final concentration | Shelf life/storage conditions |
|---|---|---|---|
| GLYI1 | 10 μL | 100 ng/mL | 48 h/4 °C |
| Ultrapure water | 990 μL | ||
| Total | 1,000 μL |
4. GLYI3 (2.5 mL)
Note: The stock solutions must be stored in plastic tubes, covered with aluminum foil.
| Reagent | Quantity | Final concentration | Shelf life/storage conditions |
|---|---|---|---|
| GLYI2 | 500 μL | 20 ng/mL | 48 h/4 °C |
| Ultrapure water | 2,000 μL | ||
| Total | 2,500 μL |
5. AMPA stock solution (1 mg/mL) (10 mL)
Note: The stock solutions must be stored in plastic tubes, covered with aluminum foil.
| Reagent | Quantity | Final concentration | Shelf life/storage conditions |
|---|---|---|---|
| AMPA analytical standard | 10 mg | 1 mg/mL | 6 months/4 °C |
| Ultrapure water | 10 mL | ||
| Total | 10 mL |
6. AMPAI1 (1 mL)
Note: The intermediate solutions must be stored in plastic tubes, covered with aluminum foil.
| Reagent | Quantity | Final concentration | Shelf life/storage conditions |
|---|---|---|---|
| AMPA stock solution | 10 μL | 10 μg/mL | 48 h/4 °C |
| Ultrapure water | 990 μL | ||
| Total | 1,000 μL |
7. AMPAI2 (1 mL)
Note: The intermediate solution must be stored in a plastic tube, covered with aluminum foil.
| Reagent | Quantity | Final concentration | Shelf life/storage conditions |
|---|---|---|---|
| AMPAI1 | 10 μL | 100 ng/mL | 48 h/4 °C |
| Ultrapure water | 990 μL | ||
| Total | 1,000 μL |
8. AMPAI3 (2.5 mL)
Note: The intermediate solution must be stored in a plastic tube, covered with aluminum foil.
| Reagent | Quantity | Final concentration | Shelf life/storage conditions |
|---|---|---|---|
| AMPAI2 | 500 μL | 20 ng/mL | 48 h/4 °C |
| Ultrapure water | 2,000 μL | ||
| Total | 2,500 μL |
9. GLY calibration curve
Note: The standard solutions for the calibration curve should be prepared in duplicate and processed on the same day as the samples. Standards of higher concentration (up to 50 ng/mL) may be prepared if necessary.
| Standard solution | Reagent | Quantity | Final concentration | Shelf life/storage conditions |
|---|---|---|---|---|
| GLY 1 | GLYI3 | 50 μL | 1 ng/mL | 48 h/4 °C |
| Ultrapure water | 950 μL | |||
| Total | 1,000 μL | |||
| GLY 2 | GLYI3 | 250 μL | 5 ng/mL | 48 h/4 °C |
| Ultrapure water | 750 μL | |||
| Total | 1,000 μL | |||
| GLY 3 | GLYI3 | 500 μL | 10 ng/mL | 48 h/4 °C |
| Ultrapure water | 500 μL | |||
| Total | 1,000 μL |
10. AMPA calibration curve
Note: The standard solutions for the calibration curve should be prepared in duplicate and processed on the same day as the samples. Standards of higher concentration (up to 50 ng/mL) may be prepared if necessary.
| Standard | Reagent | Quantity | Final concentration | Shelf life/storage conditions |
|---|---|---|---|---|
| AMPA 1 | AMPAI3 | 125 μL | 2.5 ng/mL | 48 h/4 °C |
| Ultrapure water | 875 μL | |||
| Total | 1,000 μL | |||
| AMPA 2 | AMPAI3 | 250 μL | 5 ng/mL | 48 h/4 °C |
| Ultrapure water | 750 μL | |||
| Total | 1,000 μL | |||
| AMPA 3 | AMPAI3 | 500 μL | 10 ng/mL | 48 h/4 °C |
| Ultrapure water | 500 μL | |||
| Total | 1,000 μL |
11. FMOC-Cl stock solution (50 mL)
Note: The solution must be stored in a plastic tube covered with aluminum foil.
| Reagent | Quantity | Final concentration | Shelf life/storage conditions |
|---|---|---|---|
| FMOC-Cl 99% | 0.115 g | 2.3 g/mL | 3 months/4 °C |
| Acetonitrile | 50 mL | ||
| Total | 50 mL | n/a |
12. FMOC-Cl working solution (50 mL)
Note: The solution must be stored in a plastic tube covered with aluminum foil.
| Reagent | Quantity | Final concentration | Shelf life/storage conditions |
|---|---|---|---|
| FMOC stock solution | 5 mL | 230 mg/L | 3 months/4 °C |
| Acetonitrile | 45 mL | ||
| Total | 50 mL | n/a |
13. Na2B4O7·10H2O buffer solution 0.125 M pH 9 (120 mL)
Note: The solution can be gently heated under constant stirring to dissolve the reagent completely. If precipitation is observed after some storage time, this procedure may be repeated to redissolve the precipitate. This is a very strong buffer solution; it is recommended to use 3 M HCl or another strong acid to adjust to a pH 9. The final pH of this buffer solution after preparation should be between 10 and 12.
| Reagent | Quantity | Final concentration | Shelf life/storage conditions |
|---|---|---|---|
| Na2B4O7·10H2O | 5.720 g | 0.125 M | 6 months/ambient temperature in an amber flask |
| Ultrapure water | Quantity sufficient | ||
| Total | 120 mL | n/a |
14. 3 M HCl solution (50 mL)
| Reagent | Quantity | Final concentration | Shelf life/storage conditions |
|---|---|---|---|
| HCl | 12.5 mL | 3 M | 1 year/ambient temperature in an amber flask |
| Ultrapure water | 37.5 mL | ||
| Total | 50 mL | n/a |
15. KH2PO4 buffer solution 50 mM pH 5.5 (250 mL)
Note: It is recommended to use NaOH 10% w/v (see Recipe 16) to take this buffer to pH 5.5. This solution should be immediately filtered using a 0.22 μm nylon plain filter disc after preparation.
| Reagent | Quantity | Final concentration | Shelf life/storage conditions |
|---|---|---|---|
| KH2PO4 | 1.7011 g | 50 mM | 1 week/4 °C in an amber flask |
| Ultrapure water | 250 mL | ||
| Total | 250 mL | n/a |
16. NaOH 10% w/v solution (100 mL)
| Reagent | Quantity | Final concentration | Shelf life/storage conditions |
|---|---|---|---|
| NaOH 97% | 10 g | 10% w/v | 1 year/ambient temperature in an amber flask |
| Ultrapure water | 100 mL | ||
| Total | 100 mL |
17. H3PO4 4% v/v solution (100 mL)
| Reagent | Quantity | Final concentration | Shelf life/storage conditions |
|---|---|---|---|
| H3PO4 85% | 4 mL | 4% v/v | 1 year/ambient temperature in an amber flask |
| Ultrapure water | 96 mL | ||
| Total | 100 mL | n/a |
For AH- quantification
1. MPA 10% w/v (25 mL)
| Reagent | Quantity | Final concentration | Shelf life/storage conditions |
|---|---|---|---|
| MPA ACS reagent 33.5%–36.5% | 2.5 g | 10% w/v | 3 months/4 °C in an amber flask |
| Ultrapure water | 25 mL | ||
| Total | 25 mL | n/a |
2. MPA 0.8% w/v (200 mL)
Note: This solution should be immediately filtered using a 0.22 μm nylon plain filter disc after preparation.
| Reagent | Quantity | Final concentration | Shelf life/storage conditions |
|---|---|---|---|
| MPA 10% w/v | 16 mL | 0.8% w/v | 1 week/4 °C in an amber flask |
| Ultrapure water | 184 mL | ||
| Total | 200 mL | n/a |
3. L-ascorbic acid stock solution (1 mL)
Note: The standard stock solution should be prepared on the same day as the samples.
| Reagent | Quantity | Final concentration | Shelf life/storage conditions |
|---|---|---|---|
| L-ascorbic acid 99% | 1 mg | 1 mg/mL | 24 h/4 °C |
| Ultrapure water | 1 mL | ||
| Total | 1 mL | n/a |
4. L-ascorbic acid working solution
Note: The standard working solution should be prepared on the same day as the samples.
| Reagent | Quantity | Final concentration | Shelf life/storage conditions |
|---|---|---|---|
| L-ascorbic acid stock solution | 0.2 mL | 0.1 mg/mL | 24 h/4 °C |
| MPA 0.8% w/v | 0.8 mL | ||
| Total | 1 mL | n/a |
Laboratory supplies
For GLY and AMPA quantification
1. Conical polypropylene centrifuge tubes with plastic screw cap, 15 mL, 120 mm × 17 mm (Sarstedt, catalog number: 62.554.009)
2. Aluminum foil
3. Microtip 2–200 μL (ABDOS, catalog number: P10101)
4. Microtip 100–1,000 μL (ABDOS, catalog number: P10102)
5. Microtip 1–5 mL (ABDOS, catalog number: P10144)
6. 1 mL disposable syringes, sterile (Neojet, catalog number: 12122)
7. Acrodisc® nylon syringe filters, 13 mm, 0.2 μm (Waters, catalog number: WAT200524)
8. 9 mm, Amber, screw-top vial, 12 × 32 mm with cap and pre-slit PTFE/Silicone septa (Waters, catalog number: 186000847C)
9. MAGNA® Nylon, supported plain filter disc, 0.22 μm (Osmonics Inc., catalog number: R02SP04700)
10. Chromatographic column ZORBAX Eclipse Plus C18, rapid resolution HD, 2.1 × 50 mm (1.8 μm) (Agilent, catalog number: 959757-902)
For AH- analysis
1. Microtip 2–200 μL (ABDOS, catalog number: P10101)
2. Microtip 100–1,000 μL (ABDOS, catalog number: P10102)
3. Microtip 1–5 mL (ABDOS, catalog number: P10144)
4. Spinwin® polypropylene microcentrifuge tubes, 1.5 mL (Tarsons, catalog number: 500010)
5. Nanosep® centrifugal devices 0.2 μm Bio-Inert (Pall Corporation, catalog number: ODM02C34)
6. 1 mL disposable syringes, sterile (Neojet, catalog number: 12122)
7. MAGNA® nylon, supported plain filter disc, 0.22 μm (Osmonics Inc., catalog number: R02SP04700)
8. Chromatographic column Supelcosil LC-18 3.3 cm × 4.6 mm (3 μm) (Supelco, catalog number: 58977)
9. Silica (quartz) UV-VIS spectrophotometer cuvette, 10 mm light path (Hellma, catalog number: Z600210-1EA)
Equipment
1. Refrigerated microcentrifuge (Eppendorff, model: 5415R)
2. Vortex (Willmington NC, model: 18405)
3. Automatic pipettes (1–10 μL; 2–20 μL; 20–200 μL; 100–1,000 μL; 1–5 mL) (Gilson, model: Pipetman)
4. Barnstead E-Pure water purification system (Thermo Scientific, model: D4642-33)
5. Sonicator (Branson, model: Sonifier 450)
6. HPLC with electrochemical detector (Perkin Elmer, model: Binary LC Pump 250, integrator 1022; manual injector loop 20 μL), electrochemical detector (ESA, detector model: Coulochem II; analytical cell model: 5011; guard cell model: 5020)
7. UHPLC with fluorometric detector (Waters, model: Acquity UPLC System)
8. High-precision analytical scale (Mettler, model: H6T)
9. Spectrophotometer (Beckman-Coulter, model: DU7400)
Software and datasets
1. Empower Pro (version 2154, 2005). Proprietary software from Waters Corporation. Used for equipment control and results analysis for Waters Acquity® UPLC. Used for peak area integration. It may be replaced by any software associated with an HPLC/UHPLC that has automatic or manual peak integration capabilities
2. Microsoft Excel 365 (version 2410, 2024). It may be replaced by LibreOffice or another free and open-source datasheet software
3. BioRender (https://www.biorender.com/). The following figures were created using BioRender: Graphical overview, Ostera, J. (2025) https://BioRender.com/k98g299
Procedure
文章信息
稿件历史记录
提交日期: Dec 17, 2024
接收日期: Feb 25, 2025
在线发布日期: Mar 20, 2025
出版日期: Apr 5, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Ostera, J. M., Olmos, V., Puntarulo, S., Bonetto, J. G. and Malanga, G. (2025). High-Performance Liquid Chromatography Quantification of Glyphosate, Aminomethylphosphonic Acid, and Ascorbate in Culture Medium and Microalgal Cells. Bio-protocol 15(7): e5263. DOI: 10.21769/BioProtoc.5263.
分类
植物科学 > 植物生物化学 > 其它化合物
生物化学 > 其它化合物 > 抗坏血酸盐
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link