- EN - English
- CN - 中文
Flotation Assay With Fluorescence Readout to Study Membrane Association of the Enteroviral Peripheral Membrane Protein 2C
基于荧光读数的漂浮分析法研究肠道病毒周边膜蛋白2C的膜结合特性
发布: 2025年04月05日第15卷第7期 DOI: 10.21769/BioProtoc.5261 浏览次数: 1605
评审: Jibin SadasivanQingliang ShenRan Chen
Abstract
Enteroviruses are abundant pathogens of humans and animals. Their replication is strictly dependent on the conserved, viral AAA+ ATPase 2C. 2C is an oligomerizing, peripheral membrane protein, and its low solubility as recombinant protein has hampered functional studies of the full-length, recombinant protein bound to a membrane. Here, we describe a modification of the classical, ultracentrifugation-based liposome flotation assay optimized to study the interaction of recombinant 2C with membranes and the functions of membrane-bound, full-length recombinant 2C. The assay takes advantage of the high solubility of recombinant 2C while fused to a maltose-binding protein. Removing this solubility-enhancing tag by specific protease cleavage in the presence of liposomes allows 2C to associate with membranes prior to aggregating. Fluorophore labeling of protein and liposomes allows rapid and precise quantitation of 2C’s association with membranes. This assay is adaptable to any peripheral membrane protein that can be fluorophore-labeled and expressed as a solubility-enhancing fusion protein.
Key features
• This protocol extends widely used liposome flotation assays to low-solubility peripheral membrane proteins, such as the enteroviral protein 2C.
• 2C is expressed and purified as a fusion protein with a solubility-enhancing tag, which is cleaved off in the presence of liposomes.
• Fluorophore-labeling of liposomes and protein facilitates quantitative readout of protein association with membranes.
• The protein-conjugated liposomes can also be used for other studies using, e.g., dynamic light scattering, cryo-EM, and enzymatic activity assays.
Keywords: Enterovirus (肠道病毒)Graphical overview
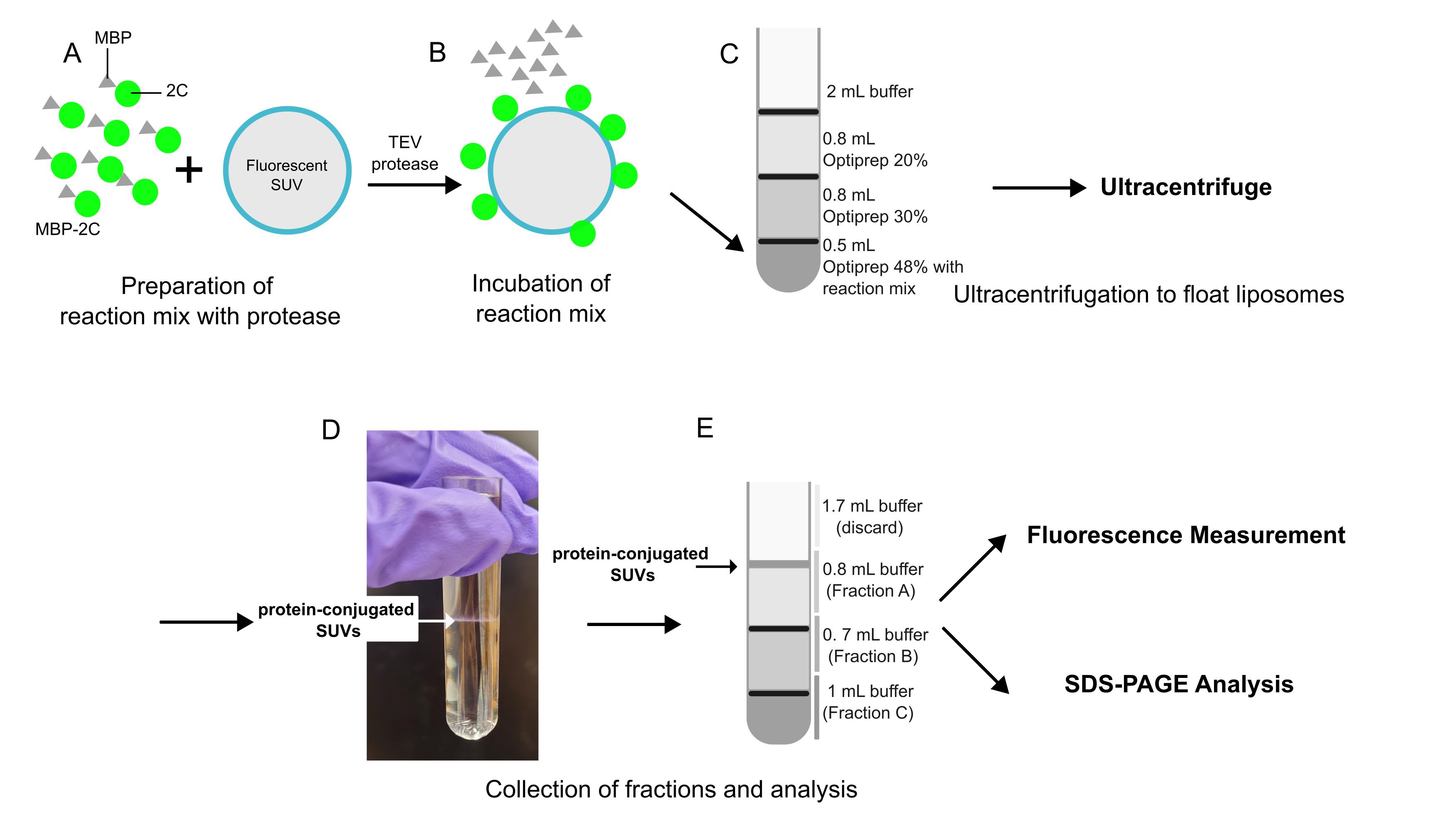
Overview of flotation assay
Background
Peripheral membrane proteins can associate with membranes through interactions with integral membrane proteins, binding to lipid headgroups, or shallow hydrophobic insertion. Understanding the factors that control their membrane association (and dissociation) is often an important step toward elucidating peripheral membrane proteins’ function. However, they frequently have limited solubility, which hampers their study in biochemical assays relying on purified proteins. To circumvent protein insolubility problems, researchers sometimes resort to strategies that increase protein solubility, such as truncation of the membrane-binding part of the protein, expression of the protein fused with a solubility-enhancing tag, or purification in the presence of detergents [1,2]. Though such strategies allow researchers to study the protein, they all involve various compromises. A key protein in the replication of enteroviruses is the peripheral membrane protein 2C, a conserved AAA+ ATPase. 2C has previously been studied using the solubility-enhancing methods described above. Here, we describe a variation of the liposome flotation assay [3] that allows the study of recombinant, full-length poliovirus 2C bound to membranes and the use of these protein-conjugated vesicles for activity assays, cryo-EM, etc. This protocol uses full-length 2C protein, which is purified as a fusion protein with an N-terminal maltose-binding protein (MBP) tag. This tag is proteolytically removed from 2C in the presence of liposomes, which allows the full-length protein to associate with a membrane before precipitating. An additional advantage to this version of the flotation assay includes quantitation through fluorescent labeling of protein and lipid. The protein-coated liposomes can also be used for other assays without involving the flotation step. Limitations of the method include the requirement of a costly ultracentrifuge with a suitable swing-out rotor as well as a fluorimeter or fluorescence-capable plate reader for fluorescence quantitation.
Materials and reagents
Reagents
1. 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoethanolamine (POPE) (Avanti Polar Lipids, catalog number: 850757P-25 mg)
2. 1-palmitoyl-2-oleoyl-glycero-3-phosphocholine (POPC) (Avanti Polar Lipids, catalog number: 850457-25 mg)
3. 1,2-Dioleoyl-sn-glycero-3-phosphoethanolamine labeled with Atto 647N (DOPE-Atto647N) (Sigma-Aldrich, catalog number: 42247-1 mg)
4. Atto 488 maleimide (Sigma-Aldrich, catalog number: 28562-1 mg)
5. OptiPrepTM density gradient medium (Sigma-Aldrich, catalog number: D1556)
6. Dithiothreitol (DTT) (Thermo ScientificTM, catalog number: R0862)
7. Tris, hydrochloride (Tris-HCl), molecular biology grade (Millipore, catalog number: 648317)
8. Potassium acetate (Sigma-Aldrich, catalog number: 236497)
9. Tris(hydroxypropyl)phosphine (THP) (Millipore, catalog number: 598250)
10. Trichloroacetic acid (Sigma-Aldrich, catalog number: T6399-100G)
11. Acetone (Sigma-Aldrich, catalog number: 179124-500ML)
12. 4× Laemmli sample buffer (Bio-Rad, catalog number: 1610747)
13. TEV protease (2 mg/mL in 50 mM Tris-HCl pH 8.0, 100 mM NaCl, 10% glycerol, 0.2 mM THP, and 300 mM imidazole (prepared in-house but can also be bought from Merck, catalog number T4455-1KU)
14. Chloroform (Honeywell, catalog number: C2432-1L)
15. Laboratory nitrogen gas
16. Water-free dimethyl sulfoxide (DMSO) (Sigma-Aldrich, catalog number: D8418-50ML)
17. 4%–20% Mini-PROTEAN® TGX Stain-FreeTM gels, 10 well, 50 μL, with Precision Plus ProteinTM unstained standards (Bio-Rad, catalog number: 17000436)
Solutions
1. Flotation buffer (see Recipes)
2. Quenching mix (see Recipes)
3. OptiPrep 48 (see Recipes)
4. OptiPrep 30 (see Recipes)
5. OptiPrep 20 (see Recipes)
6. TCA solution (see Recipes)
7. High pH 1× Laemmli buffer (see Recipes)
Recipes
1. Flotation buffer
50 mM Tris-HCl (pH 7.4), 150 mM potassium acetate, and 0.1 mM THP
2. Quenching mix
100 mM DTT in flotation buffer
3. OptiPrep 48
16 mL of OptiPrepTM density gradient medium (stock of 60% w/v) and 4 mL of flotation buffer
4. OptiPrep 30
10 mL of OptiPrepTM density gradient medium (stock of 60% w/v) and 10 mL of flotation buffer
5. OptiPrep 20
6.7 mL of OptiPrepTM density gradient medium (stock of 60% w/v) and 13.3 mL of flotation buffer
6. TCA solution
50 g of TCA in 50 mL of distilled water
7. High pH 1× Laemmli buffer
250 μL of Tris-HCl (pH 8.5) (500 mM), 500 μL of distilled water, and 250 μL of 4× Laemmli buffer
Laboratory supplies
1. Open-top thin-wall ultra-clear tube 4 mL, 11 × 60 mm (Beckman Coulter, catalog number: 344062)
2. BRAND® microcentrifuge tube, 2 mL with lid, transparent (Millipore Sigma, catalog number: Z628034-500EA)
3. Corning® 384-well solid black (Fisher Scientific, catalog number: 09-761-86)
4. Corning® 50 mL centrifuge tubes (Millipore Sigma, catalog number: CLS430828-100EA)
5. Corning® 15 mL centrifuge tubes (Millipore Sigma, catalog number: CLS430791-500EA)
6. Fisherbrand® borosilicate glass tubes 12 × 75 mm (Fisher Scientific, catalog number: 14-961-26)
7. P1000, P200, and P10 pipettes
8. Positive displacement digital microdispenser, 50 μL (Drummond Scientific Company, catalog number: 3-000-550)
9. Positive displacement digital microdispenser, 50 μL (Drummond Scientific Company, catalog number: 3-000-575)
10. Drummond® microdispenser replacement tubes, 50 μL (Drummond Scientific Company, catalog number: 3-000-250-G)
11. Drummond® microdispenser replacement tubes, 100 μL (Drummond Scientific Company, catalog number: 3-000-275-G)
12. Superose® 6 10/300 GL (Cytiva, catalog number: 17-5172-01)
Equipment
1. SW 60 Ti swinging-bucket rotor (Beckman Coulter, catalog number: 335650)
2. Ultracentrifuge (Beckman Coulter, model: OptimaTM L-90K)
3. Fluorescence plate reader (BMG Labtech, model: CLARIOstar Plus)
4. Centrifuge (Eppendorf, model: 5424R)
5. ÄKTATM pure 25 (Cytiva) or equivalent FPLC system
6. Vacuum oven (Fisher Scientific, model: Vacutherm®)
7. Q500 Sonicator® with microtip probes (Qsonica, catalog number: 4418)
Software and datasets
1. GraphPad Prism v10.0.1
Procedure
文章信息
稿件历史记录
提交日期: Jan 12, 2025
接收日期: Mar 2, 2025
在线发布日期: Mar 18, 2025
出版日期: Apr 5, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/).
如何引用
Shankar, K., Lin, Y. and Carlson, L. (2025). Flotation Assay With Fluorescence Readout to Study Membrane Association of the Enteroviral Peripheral Membrane Protein 2C. Bio-protocol 15(7): e5261. DOI: 10.21769/BioProtoc.5261.
分类
微生物学 > 微生物生物化学 > 蛋白质 > 相互作用
生物化学 > 蛋白质 > 相互作用 > 蛋白质-脂质相互作用
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link