- EN - English
- CN - 中文
Ex Vivo Measurement of Stable Isotope-Labeled Fatty Acid Incorporation Into Phospholipids in Isolated Mice Muscle
离体条件下稳定同位素标记脂肪酸在分离小鼠骨骼肌磷脂中的掺入测定
发布: 2025年04月05日第15卷第7期 DOI: 10.21769/BioProtoc.5257 浏览次数: 1845
评审: Chiara AmbrogioOm Prakash NarayanIstvan Stadler
Abstract
With the advancement of liquid chromatography–mass spectrometry (LC–MS/MS), the quantification of glycerophospholipid (PL) molecules has become more accessible, leading to the discovery of numerous enzymes responsible for determining the acyl groups attached to these molecules. Metabolic tracer experiments using radioisotopes and stable isotopes are powerful tools for defining the function of metabolic enzymes and metabolic flux. We have established an ex vivo muscle experimental system using stable isotope–labeled fatty acids to evaluate fatty acid incorporation into PL molecules. Here, we describe a method to incorporate fatty acids with stable isotope labels into excised skeletal muscle and detect the PL molecules containing labeled acyl chains by LC–MS/MS.
Key features
• Quantify the metabolism of fatty acids into phospholipid acyl chains.
• Enable measurements in excised muscle samples.
• Assess the effects of genetic recombination of acyltransferases.
Keywords: Stable isotope tracer (稳定同位素示踪)Graphical overview
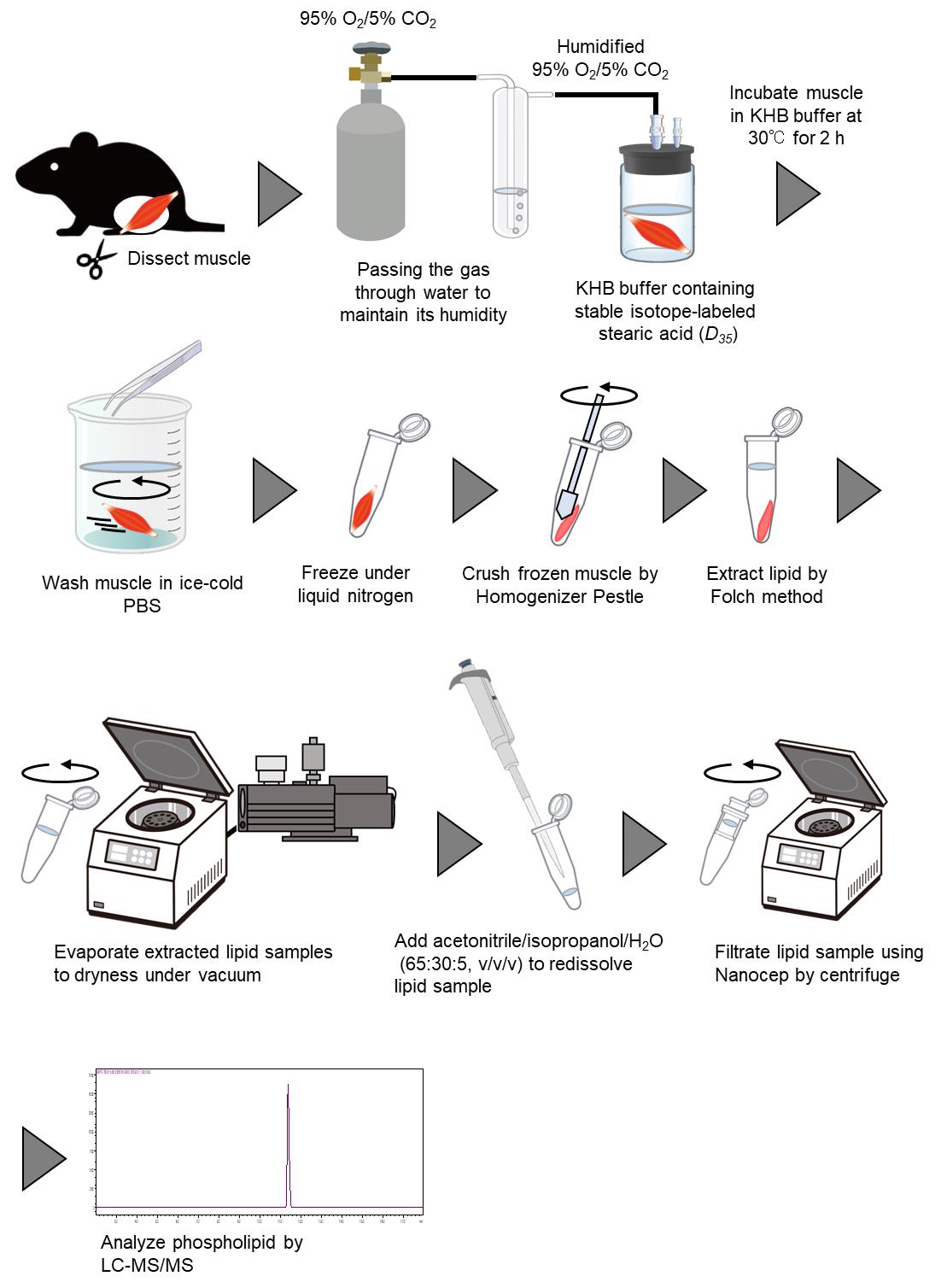
Ex vivo measurement of stable isotope–labeled fatty acid incorporation into phospholipids in isolated mice muscle
Background
Glycerophospholipids (PL) are major constituents of cellular membranes, containing two acyl chains and a polar head. Numerous varieties of PL molecules exist depending on the combination of acyl-chain species and polar heads. Various types of PLs, such as phosphatidylcholine (PC), phosphatidylethanolamine (PE), phosphatidylserine (PS), phosphatidylinositol (PI), and phosphatidylglycerol (PG), are synthesized from glycerol-3-phosphate (G3P) via the de novo pathway (Kennedy pathway) [1]. Acyl-chain species bound to PLs produced in the Kennedy pathway are remodeled by phospholipases and lysophospholipid acyltransferases (LPLATs) [2]. This remodeling pathway, called the Lands’ cycle, is essential for generating PL species diversity and asymmetric distribution of acyl chains [3].
Acyl chain species in PL molecules were traditionally quantified by the measurement of extracted fatty acids using gas chromatography–mass spectrometry (GC–MS) after hydrolyzation of PLs. Thus, measuring individual PL molecules one by one was challenging. In recent years, advancements in lipid molecule separation technology by liquid chromatography (LC) and improved detection sensitivity of MS have made it possible to measure lipid molecules exceeding 5,000 species [4,5]. Mass spectrometers can detect isotope peaks, enabling their use in combination with stable isotopes such as deuterium (2H) and 13C for tracking specific compounds in experiments. Tracking PL remodeling using deuterium-labeled palmitic acid (16:0-D31-FFA) and stearic acid (18:0-D35-FFA) has been reported in cultured cell experiments [6]. However, examples of in vivo or ex vivo PL metabolism tracer experiments are limited, highlighting the need for the development of new methods. Typically, metabolic tracers for carbohydrates and lipids in skeletal muscle are evaluated by incubating isolated skeletal muscle with radiolabeled substrates and measuring the radioactivity of the extracted components [7,8]. However, experiments using radiolabeled compounds cannot measure PL molecules with defined bound acyl group molecular species, as is possible with LC–MS/MS measurements, even though thin-layer chromatography can be used to measure the radioactivity of each PL class, such as PC and PE, with high sensitivity. Therefore, PLs labeled with stable isotopes need to be quantified by mass spectrometry. In addition, ex vivo studies allow organ-specific assessment and reduce the use of stable isotope compounds compared to in vivo studies. Satellite cells isolated from mice differentiate into a mixture of fast and slow muscle fibers, making fast and slow muscle-specific analysis difficult in an in vitro experimental system. An ex vivo experimental system using fast- and slow-twitch muscle excised from mice allows the evaluation of skeletal muscle-specific phospholipid metabolism and overcomes the above problems.
This article provides a detailed method for measuring the incorporation of stable isotope–labeled fatty acids into PLs in isolated skeletal muscle. Although the remodeling of the acyl group in PL measured by this protocol has been validated in a specific LPLAT (KO) model, a limitation of this protocol is that the contribution of Kennedy pathway cannot be ignored. Although the methods described in this article are based on excised skeletal muscle, direct injection of stable-isotope compounds into skeletal muscle may be applicable for in vivo evaluation.
Materials and reagents
Biological materials
1. C57BL/6J mice at 8–12 weeks old
Note: At least five mice are recommended.
Reagents
1. Stearic acid (D35, 98%) (Cambridge Isotope Laboratories, catalog number: DLM-379-1)
2. Fatty acid–free bovine serum albumin (BSA) (Wako, catalog number: 013-25773)
3. Chloroform (Wako, catalog number: 038-02601)
4. Methanol (Wako, catalog number: 134-14523)
5. 1, 2-diheptadecanoyl-sn-glycero-3-phosphocholine (17:0-PC) (Avanti Polar Lipids, catalog number: 850360)
6. 1, 2-dilauroyl-sn-glycero-3-phosphoethanolamine (12:0-PE) (Avanti Polar Lipids, catalog number: 850702)
Note: Dissolve 17:0-PC and 12:0-PE in a chloroform/methanol mixture (2:1) to a final concentration of 10 mg/mL. After dissolution, store the solution at -30 °C or below to prevent volatilization of the organic solvents.
7. Butylhydroxytoluene (BHT) (Wako, catalog number: 029-07392)
8. 2-Propanol (Wako, catalog number: 164-25533)
9. Ultrapure water (Wako, catalog number: 210-01303)
10. Acetonitrile (Wako, catalog number: 018-19853)
11. Ammonium formate (Sigma-Aldrich, catalog number: 70221)
12. Formic acid (Wako, catalog number: 067-04531)
13. Sodium chloride (NaCl) (Wako, catalog number: 191-01665)
14. Potassium chloride (KCl) (Wako, catalog number: 163-03545)
15. Calcium chloride dihydrate (CaCl2·2H2O) (Wako, catalog number: 039-00431)
16. Potassium dihydrogen phosphate (KH2PO4) (Wako, catalog number: 169-04245)
17. Magnesium sulfate heptahydrate (MgSO4·7H2O) (Wako, catalog number: 131-00405)
18. Glucose (Wako, catalog number: 049-31165)
19. Sodium hydrogen carbonate (NaHCO3) (Wako, catalog number: 191-01305)
Solutions
1. Krebs–Henseleit buffer (KHB) (see Recipes)
2. Mobile phase A (see Recipes)
3. Mobile phase B (see Recipes)
Recipes
1. 1 L Krebs–Henseleit buffer (KHB)
| Reagent | Final concentration (mM) | Quantity (g) |
|---|---|---|
| NaCl | 125 | 7.283 |
| KCl | 5.3 | 0.395 |
| CaCl2·2H2O | 1.9 | 0.279 |
| KH2PO4 | 1.3 | 0.171 |
| MgSO4·7H2O | 1.3 | 0.309 |
| Glucose | 5 | 0.9 |
Note: A NaHCO3 stock solution prepared to a final concentration of 1.3% and saturated with CO2 gas (sealed with liquefied CO2 for 1 h) can be used for up to one month when stored at 4 °C in a sealed container.
Caution: As various organic solvents are used for lipid extraction and as LC–MS mobile phase, they should be handled in a safety cabinet to prevent inhalation.
Caution: The KHB buffer should be used within one week. KHB buffer containing NaHCO3, BSA, and stable isotope–labeled stearic acid (D35) should only be used on the day of preparation.
2. Mobile phase A
H2O/acetonitrile (60:40, v/v)
10 mM ammonium formate
0.1% formic acid
3. Mobile phase B
Isopropanol/acetonitrile (90:10, v/v)
10 mM ammonium formate
0.1% formic acid
Laboratory supplies
1. 20 mL glass reaction vial (IWAKI, catalog number: 1890CV20)
2. Homogenizer pestle (BIO-BIK, catalog number: 1005-39)
3. Nanocep with 0.45 μm wwPTFE (PALL, catalog number: ODPTEF04C35)
4. SecurityGuard Guard Cartridge kit (Phenomenex, catalog number: KJ0-4282)
5. Accucore RP-MS column (2.6 μm, 2.1 mm × 50 mm) (Thermo ScientificTM, catalog number: 17626-012105)
Equipment
1. Anesthetic apparatus for small animals (Shinano, model: SN-487-0T AS1)
2. Thermostatic water bath (Thermal Chemical, model: DB-42)
3. Centrifugal evaporator (EYELA, model: CVE-3000)
4. Ultrasonic bath (BRANSON, model: Yamato1510)
5. Triple quadrupole mass spectrometer (Shimadzu, model: LCMS-8040)
6. Binary pump (Shimadzu, model: LC-30AD)
7. Auto sampler (Shimadzu, model: SIL-30AC)
8. Column oven (Shimadzu, model: CTO-20AC)
9. N2 gas supplier (ANEST IWATA, model: 24FD)
10. EM oil-sealed rotary vane pumps (Edwards, model: E2M28)
Software
1. LabSolutions LCMS (Shimadzu, version 5.65)
Procedure
文章信息
稿件历史记录
提交日期: Dec 25, 2024
接收日期: Feb 27, 2025
在线发布日期: Mar 13, 2025
出版日期: Apr 5, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Sato, T. and Miura, S. (2025). Ex Vivo Measurement of Stable Isotope-Labeled Fatty Acid Incorporation Into Phospholipids in Isolated Mice Muscle. Bio-protocol 15(7): e5257. DOI: 10.21769/BioProtoc.5257.
- Sato, T., Umebayashi, S., Senoo, N., Akahori, T., Ichida, H., Miyoshi, N., Yoshida, T., Sugiura, Y., Goto-Inoue, N., Kawana, H., et al. (2023). LPGAT1/LPLAT7 regulates acyl chain profiles at the sn-1 position of phospholipids in murine skeletal muscles. J Biol Chem. 299(7): 104848. https://doi.org/10.1016/j.jbc.2023.104848
分类
生物化学 > 脂质 > 膜脂
细胞生物学 > 细胞新陈代谢 > 脂质
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link