- EN - English
- CN - 中文
Monitoring Changes in Intracellular Chloride Levels Using the FRET-Based SuperClomeleon Sensor in Organotypic Hippocampal Slices
利用基于 FRET 的 SuperClomeleon 传感器监测器官型海马切片中细胞内氯离子水平变化
发布: 2025年03月05日第15卷第5期 DOI: 10.21769/BioProtoc.5229 浏览次数: 2780
评审: Marco Di GioiaThirupugal GovindarajanAndrea GramaticaDavide Pallucci
Abstract
The reduction in intracellular neuronal chloride concentration is a crucial event during neurodevelopment that shifts GABAergic signaling from depolarizing to hyperpolarizing. Alterations in chloride homeostasis are implicated in numerous neurodevelopmental disorders, including autism spectrum disorder (ASD). Recent advancements in biosensor technology allow the simultaneous determination of intracellular chloride concentration of multiple neurons. Here, we describe an optimized protocol for the use of the ratiometric chloride sensor SuperClomeleon (SClm) in organotypic hippocampal slices. We record chloride levels as fluorescence responses of the SClm sensor using two-photon microscopy. We discuss how the SClm sensor can be effectively delivered to specific cell types using virus-mediated transduction and describe the calibration procedure to determine the chloride concentration from SClm sensor responses.
Key features
• Calibration and use of the ratiometric chloride sensor SuperClomeleon to measure intracellular chloride levels in mouse organotypic hippocampal slices.
• SuperClomeleon sensor expression in pyramidal cells or GABAergic interneurons using adeno-associated viruses.
Keywords: SuperClomeleon (SuperClomeleon)Graphical overview
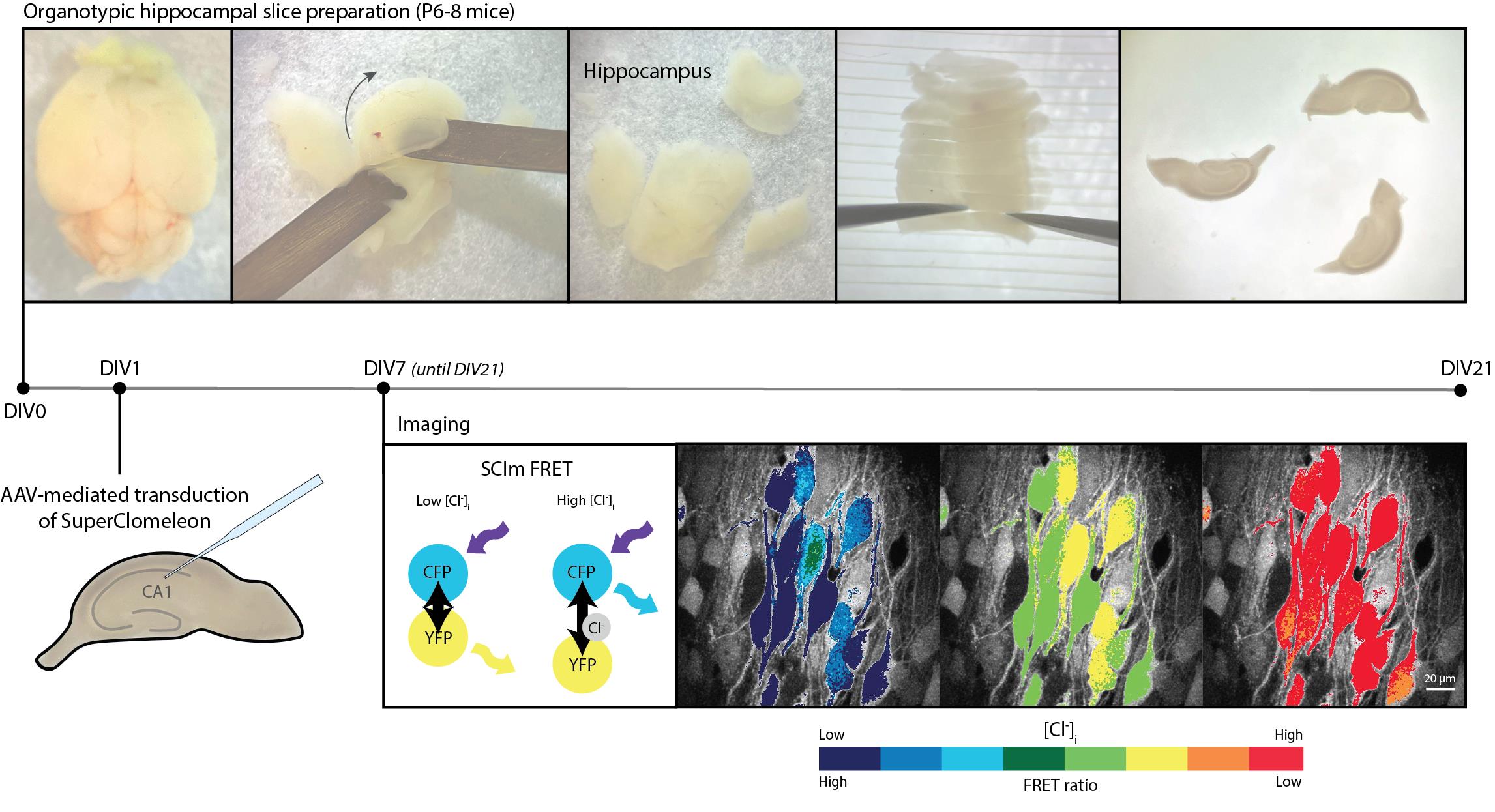
Background
Chloride ions are involved in the regulation of many basic cellular properties, including cell volume, intracellular pH, and membrane potential. Defects in chloride homeostasis occur in many diseases, including neurodevelopmental disorders. In neurons, chloride mediates synaptic signaling. While GABA is the main inhibitory neurotransmitter in the adult brain, during early development, GABAergic signaling is excitatory, which aids proper wiring of the developing brain [1,2]. The transition of GABAergic action from depolarizing to hyperpolarizing later in development is called the GABA shift. A disturbance of the GABA shift is implicated in various neurodevelopmental disorders [2–5]. The GABA shift is the result of a delayed expression of the chloride exporter KCC2 in developing neurons, while immature neurons already express the chloride importer NKCC1 [1]. As a consequence, intracellular chloride concentrations are high in immature neurons, while healthy adult neurons maintain low chloride levels. To characterize the GABA shift during brain development, or to assess changes in chloride homeostasis in the context of diseases, a reliable method to determine intracellular chloride concentrations is indispensable.
A reliable method to determine the intracellular chloride concentration in individual neurons is performing perforated patch clamp recordings using selective cation-permeable ionophores [6,7]. This technique is technically difficult to perform and time-intensive, resulting in a relatively low yield. As there is ample variability between individual cells, a high number of experiments is required in order to draw conclusions on the population level. Recent technological advancements in biosensors, more specifically in chloride sensors, are now providing a promising high-throughput alternative, in which intracellular chloride levels can be monitored without interference of the intracellular milieu. There are several chloride sensors currently under development, each with specific advantages and disadvantages [8].
Early chemical chloride sensors lacked the ability to target specific groups of cells, while single color-based genetically encoded chloride sensors are sensitive to variations in the local concentration of the sensor [9]. Fluorescence resonance energy transfer (FRET)-based sensors circumvent these issues as their expression can be targeted to specific cell types using the Cre-Lox system and their ratiometric properties allow the determination of chloride concentration independently of sensor concentration [8,9]. The FRET sensor SuperClomeleon (SClm) is an improved version of the first genetically encoded ratiometric chloride sensor Clomeleon. It provides enhanced chloride sensitivity and is well-suited for the simultaneous imaging of chloride levels in many neurons [8,10,11]. SClm contains two fluorescent proteins, Cerulean (CFP mutant) and Topaz (YFP mutant), which are joined together by a flexible linker region [11]. FRET occurs between the CFP donor and the YFP acceptor when chloride is not bound, whereas no FRET occurs when chloride is bound to the sensor. In this way, the FRET ratio (YFP/CFP fluorescence intensity) indicates the intracellular chloride concentration. The SClm sensor has been successfully employed to study the contribution of NKCC1 and the WNK pathway to chloride homeostasis in neuronal cultures [12], to determine steady-state intracellular chloride concentration in cortical neurons in vivo [13], and to demonstrate chloride microdomains in hippocampal pyramidal neurons [14], illustrating its broad applicability. We recently used SClm to monitor developmental changes in intraneuronal chloride concentration in organotypic hippocampal slices [15]. In the current protocol, we provide a detailed description of how to optimize and calibrate SClm for determining intracellular chloride concentration in developing organotypic hippocampal slices. SClm expression in pyramidal cells and interneurons was achieved via virus-mediated transduction. We provide a step-by-step description of the preparation of slice cultures and virus injection and describe the two-photon microscopy and image analysis. Lastly, we discuss how to properly calibrate the sensor to determine intracellular chloride concentrations from measured FRET ratios.
Materials and reagents
Biological materials
1. C57BL/6 wild-type mice (The Jackson Laboratory, strain #000664)
2. C57BL/6 VGAT-Cre mice B6J.129S6(FVB)-Slc32a1tm2(cre)Lowl/MwarJ (The Jackson Laboratory, strain #028862)
Note: VGAT is the vesicular GABA transporter (encoded by the Slc32a1 gene). VGAT-Cre mice allow targeted expression of the SuperClomeleon sensor in (VGAT-expressing) interneurons.
3. Floxed SuperClomeleon construct under the control of synapsin promotor (a gift from prof. dr. Thomas Kuner, Heidelberg University, Germany)
4. Non-floxed SuperClomeleon construct under the control of the synapsin promotor (a gift from Kevin Staley, Massachusetts General Hospital, Boston, MA)
Note: Different SClm constructs are now commercially available from the Addgene website: https://www.addgene.org/browse/article/28244285/
Reagents
1. Calcium chloride (CaCl2) (Merck, catalog number 102382)
2. D-(+)-glucose (Sigma-Aldrich, catalog number: G7528)
3. Ethylene glycol-bis(β-aminoethyl ether)-N,N,N',N'-tetraacetic acid (EGTA) (Sigma-Aldrich, catalog number: E4378)
4. Hanks’ balanced salt solution (HBSS) (Gibco, catalog number: 24020-091)
5. HEPES (Sigma-Aldrich, catalog number: H4034)
6. Horse serum (Gibco, catalog number: 26050-088)
7. K-gluconate (Sigma-Aldrich, catalog number: P1847)
8. Kynurenic acid (Sigma-Aldrich, catalog number: K3375)
9. Magnesium chloride (MgCl2) (Sigma-Aldrich, catalog number: M2670)
10. Magnesium sulfate (MgSO4) (Sigma-Aldrich, catalog number: 63138)
11. Mg(gluconate)2 (Sigma-Aldrich, catalog number: G9130)
12. MgATP (Carbosynth, catalog number: NA44288)
13. Minimal essential medium 1× (MEM) (Gibco, catalog number: 11575-032)
14. Na2-phosphocreatine (Sigma-Aldrich, catalog number: P7936)
15. NaF (Sigma-Aldrich, catalog number: 201154)
16. Na-gluconate (Sigma-Aldrich, catalog number: S2054)
17. NaGTP (Sigma-Aldrich, catalog number: G8877)
18. Nigericin (Sigma-Aldrich, catalog number: N7143)
19. Phosphate buffered saline (PBS), pH 7.2, 1× (Gibco, catalog number: 20012027)
20. Potassium chloride (KCl) (Sigma-Aldrich, catalog number: P5405)
21. Potassium fluoride (KF) (Thermo Scientific, catalog number: 42216)
22. Potassium phosphate monobasic (KH2PO4) (Sigma-Aldrich, catalog number: P5655)
23. Sodium chloride (NaCl) (Sigma-Aldrich, catalog number: S7653)
24. Sodium dihydrogen phosphate (NaH2PO4) (Merck, catalog number: A977446)
25 Sodium hydrogen carbonate (NaHCO3) (Merck, catalog number: 106329)
26. Tributyltin acetate (Merck, catalog number: 821650)
27. Trolox (Acros Organics, catalog number: 218940250)
Solutions
1. Gey’s balanced salt solution (GBSS) (see Recipes)
2. Culture medium (see Recipes)
3. Artificial cerebrospinal fluid (ACSF) (see Recipes)
4. High chloride solution (see Recipes)
5. Low chloride solution (see Recipes)
6. KF quenching solution (see Recipes)
Recipes
1. Gey’s balanced salt solution (GBSS) (50 mL)
Note: Osmolarity should be 325 ± 5 mOsm. Add sucrose to increase osmolarity if needed. pH should be 7.3–7.4.
| Reagent | Final concentration |
| NaCl | 137 mM |
| KCl | 1.5 mM |
| CaCl2 | 1.5 mM |
| MgCl2 | 1 mM |
| MgSO4 | 0.3 mM |
| KH2PO4 | 0.2 mM |
| Na2HPO4 | 0.85 mM |
| D-(+)-glucose | 25 mM |
| HEPES | 12.5 mM |
| Kynurenic acid | 1 mM |
2. Culture medium (50 mL)
Note: Osmolarity should be 325 ± 5 mOsm. pH should be 7.3–7.4. Culture medium can be prepared prior to use and kept in the fridge for 2–3 weeks.
| Reagent | Final concentration |
|---|---|
| MEM | 48% |
| HBSS | 25% |
| Horse serum | 25% |
| D-(+)-glucose | 25 mM |
| HEPES | 12.5 mM |
3. Artificial cerebrospinal fluid (ACSF) (500 mL)
Note: Usually, two 10× stock solutions are made: one with the first four salts and one containing NaH2PO4/NaHCO3. On the day ACSF is used, the stock solutions are diluted, and glucose and Trolox are added. Trolox is hard to dissolve; thoroughly stir using a magnetic bean before use for at least 1 h. Ensure that the osmolarity is 310 ± 10 mOsm.
| Reagent | Final concentration |
|---|---|
| NaCl | 126 mM |
| KCl | 3 mM |
| CaCl2 | 2.5 mM |
| MgCl2 | 1.3 mM |
| NaHCO3 | 26 mM |
| NaH2PO4 | 1.25 mM |
| D-(+)-glucose | 20 mM |
| Trolox | 1 mM |
4. High chloride stock (200 mL)
| Reagent | Final concentration |
|---|---|
| KCl | 105 mM |
| NaCl | 48 mM |
| HEPES | 10 mM |
| D-(+)-glucose | 20 mM |
| Na-EGTA | 2 mM |
| MgCl2 | 4 mM |
5. Low chloride stock (200 mL)
| Reagent | Final concentration |
|---|---|
| K-gluconate | 105 mM |
| Na-gluconate | 48 mM |
| HEPES | 10 mM |
| D-(+)-glucose | 20 mM |
| Mg(gluconate)2 | 4 mM |
6. KF quenching solution (100 mL)
| Reagent | Final concentration |
|---|---|
| KF | 105 mM |
| NaF | 48 mM |
| HEPES | 10 mM |
| D-(+)-glucose | 20 mM |
| Na-EGTA | 2 mM |
| Mg(gluconate)2 | 4 mM |
Laboratory supplies
1. Platinum-coated blades (Safety Razor Co. Ltd., Feather, catalog number: 45176517)
2. Stainless steel surgical blades, No. 23 (Swann-Morton, catalog number: 0310)
3. Qualitative filter paper (VWR European, catalog number: 516-0350)
4. 0.2 μm syringe filter (Fisherbrand, catalog number: 15206869)
5. 0.45 μm PTFE membrane (Omnipore, catalog number: JHWP04700)
6. Cell culture inserts with 0.4 μm PTFE membrane (Millicell, catalog number: PICM0RG50)
7. 6-well cell culture plate (Corning Incorporated, catalog number: 3516)
8. Petri dish 94 × 16 mm (Greiner Bio-One, catalog number: 633181)
9. Petri dish 60 × 15 mm (Greiner Bio-One, catalog number 628161)
10. Thin wall glass capillaries (World Precision Instruments, catalog number: TW100F-4)
11. Microloader pipette tips (Eppendorf, catalog number: 5242956003)
Equipment
A. Organotypic hippocampal slice preparation and slice injection
1. Wide-field light microscope (Kern Optics, model: OZL468)
2. Flow cabinet (Clean Air, catalog number: EN 12469)
3. Class 5 open flow hood (Procleanroom)
4. Bead sterilizer (Simon Keller, Steri 250, catalog number: 6.285 884)
5. UV sterilizer (Mega Beauty Shop, catalog number: MBS-FMX6)
6. McIlwain tissue chopper (Caver Laboratory Engineering Co. Ltd., catalog number: MTC/2E)
7. Stereomicroscope (Leica, model: M80)
8. Microinjector (Eppendorf, model: FemtoJet 4×)
9. Micromanipulator (Narishige Japan, model: MMO-220A)
10. Pipette puller (Sutter Instrument Corporation, model: P-2000)
11. CO2 incubator (PHCBI, catalog number: MCO-170AICUVH)
B. Two-photon microscopy
Note: SClm imaging in 2D cultures can also be performed using confocal or upright microscopy.
1. Customized two-photon laser scanning microscope (Femto3D-RC, Femtonics, Budapest, Hungary) with two GaAsP photomultiplier tubes
2. Ti-Sapphire femtosecond pulsed laser (MaiTai HP, Spectra-Physics) tuned to 840 nm
3. Filter cube with dichroic beam splitter at 505 nm and band-pass filters at 470–500 nm (CFP channel) and 520–550 nm (YFP channel)
4. 4× air objective (Nikon, model: CFI Plan Apochromat Lambda D 4×/0.20)
5. 60× water immersion objective (Nikon, model: NIR Apochromat, NA 1.0)
Software and datasets
1. ImageJ/Fiji (v1.54m, December 2024)
2. GraphPad Prism (v10.1.2, December 2023)
3. Femtonics MES (v6.5, December 2020)
Procedure
文章信息
稿件历史记录
提交日期: Nov 26, 2024
接收日期: Jan 26, 2025
在线发布日期: Feb 25, 2025
出版日期: Mar 5, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- de Kater, S., Herstel, L. J. and Wierenga, C. J. (2025). Monitoring Changes in Intracellular Chloride Levels Using the FRET-Based SuperClomeleon Sensor in Organotypic Hippocampal Slices. Bio-protocol 15(5): e5229. DOI: 10.21769/BioProtoc.5229.
- Herstel, L. J., Peerboom, C., Uijtewaal, S., Selemangel, D., Karst, H. and Wierenga, C. J. (2022). Using SuperClomeleon to Measure Changes in Intracellular Chloride during Development and after Early Life Stress. eNeuro. 9(6): ENEURO.0416–22.2022. https://doi.org/10.1523/eneuro.0416-22.2022
分类
神经科学 > 细胞机理
细胞生物学 > 细胞成像 > 双光子显微镜
生物物理学 > 显微技术 > 双光子激光扫描显微镜
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link