- EN - English
- CN - 中文
Puromycin Proximity Ligation Assay (Puro-PLA) to Assess Local Translation in Axons From Human Neurons
嘌呤霉素近邻连接检测(Puro-PLA)评估人神经元轴突局部翻译
发布: 2025年03月05日第15卷第5期 DOI: 10.21769/BioProtoc.5224 浏览次数: 3282
评审: Marion HoggYusuke TominaJosé M. Dias
Abstract
Local mRNA translation in axons is crucial for the maintenance of neuronal function and homeostasis, particularly in processes such as axon guidance and synaptic plasticity, due to the long distance from axon terminals to the soma. Recent studies have shown that RNA granules can hitchhike on the surface of motile lysosomal vesicles, facilitating their transport within the axon. Accordingly, disruption of lysosomal vesicle trafficking in the axon, achieved by knocking out the lysosome–kinesin adaptor BLOC-one-related complex (BORC), decreases the levels of a subset of mRNAs in the axon. This depletion impairs the local translation of mitochondrial and ribosomal proteins, leading to mitochondrial dysfunction and axonal degeneration. Various techniques have been developed to visualize translation in cells, including translating RNA imaging by coat protein knock-off (TRICK), SunTag, and metabolic labeling using the fluorescent non-canonical amino acid tagging (FUNCAT) systems. Here, we describe a sensitive technique to detect newly synthesized proteins at subcellular resolution, the puromycin proximity ligation assay (Puro-PLA). Puromycin, a tRNA analog, incorporates into nascent polypeptide chains and can be detected with an anti-puromycin antibody. Coupling this method with the proximity ligation assay (PLA) allows for precise visualization of newly synthesized target proteins. In this article, we describe a step-by-step protocol for performing Puro-PLA in human induced pluripotent stem cell (iPSC)-derived neuronal cultures (i3Neurons), offering a powerful tool to study local protein synthesis in the axon. This tool can also be applied to rodent neurons in primary culture, enabling the investigation of axonal protein synthesis across species and disease models.
Key features
• Establishment of quantitative local translation assay in axons of human iPSC-derived neurons.
• Microscopy-based direct visualization of local translation events in neurons.
• Puro-PLA is a sensitive method for detecting new protein synthesis occurring within minutes in neurons, enabling precise temporal analysis of translation dynamics.
Keywords: Human i3Neurons (人 i3 神经元)Graphical overview
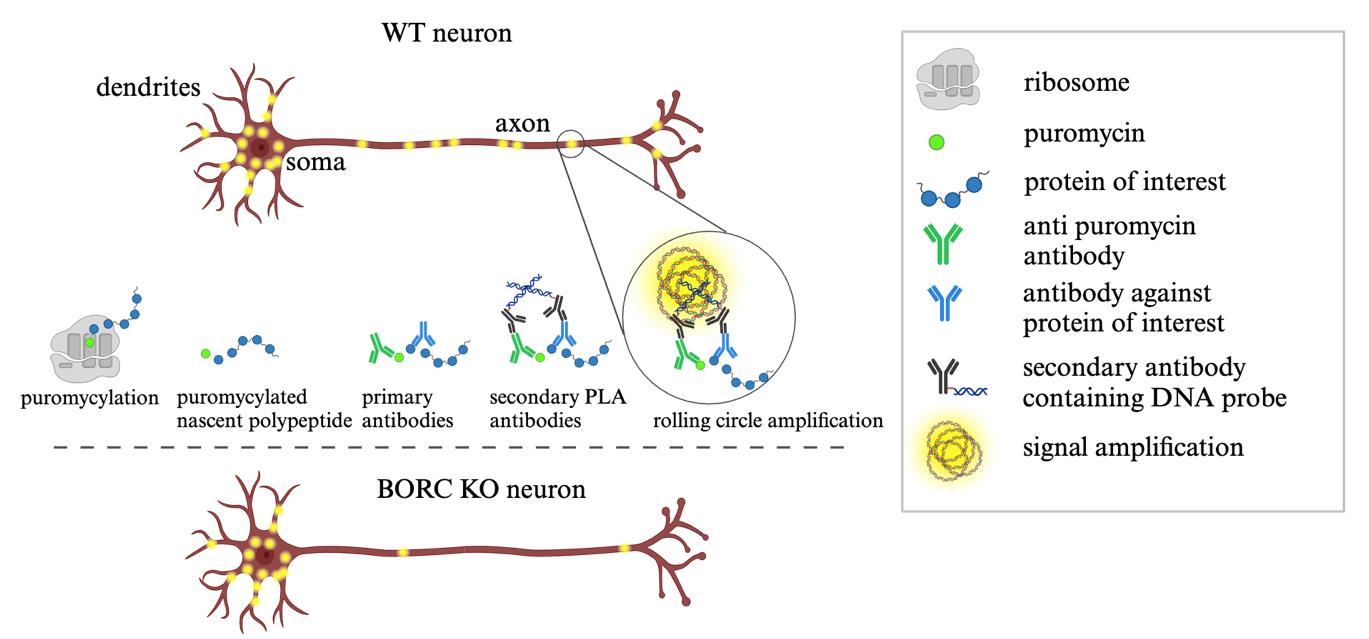
Schematic representation of puromycin proximity ligation assay (Puro-PLA) steps. Steps involved in performing Puro-PLA reaction in wildtype (WT) and BORC knock-out (KO) neurons. WT axons exhibit abundant puromycin-labeled translation sites, represented as yellow fluorescent dots along the axon, indicating active protein synthesis. In contrast, the axon of BORC KO neurons displays fewer fluorescent dots, reflecting reduced local translation due to impaired transport of RNA granules in association with lysosomal vesicles.
Background
Neurons are highly polarized cells with long neurites, particularly axons, that transmit signals over long distances. To maintain the functionality of axon terminals, the transport of axonal mRNAs and their local translation are essential. Local translation of mRNAs in axons enables a rapid, on-demand supply of proteins, offering a more efficient alternative to the slower transport of proteins from the soma. Disruptions in axonal mRNA transport and local translation have been implicated in the pathogenesis of neurodegenerative diseases like amyotrophic lateral sclerosis (ALS), frontotemporal dementia (FTD), and Alzheimer's disease (AD) [1–3]. The visualization of mRNA translation events is therefore extremely relevant and widely used to monitor local translation with both spatial and temporal resolution across different cell types. Various techniques have been developed for this purpose, including translating RNA imaging by coat protein knock-off (TRICK) [4], SunTag [5], SUnSET [6], BONCAT [7], SILAC [8], and puromycin proximity ligation assay (Puro-PLA) [9,10]. The Puro-PLA technique enables the visualization of individual molecules being translated within 10 min from the addition of puromycin. This short time minimizes the number of events, resulting in reduced background noise and facilitating the quantification of single particles. Once added, the puromycin gets incorporated into the nascent peptides, causing them to be displaced from the ribosome. This causes the premature termination of nascent proteins and labels them with puromycin residues [11]. The puromycin and the protein of interest can then be recognized by specific primary antibodies. A pair of secondary antibodies containing DNA probes then binds to the primary antibodies and amplifies the signal (Graphical overview). The Puro-PLA technique is highly sensitive, allowing for the detection of low-abundance proteins. Additionally, it offers high specificity by relying on proximity ligation of protein-specific antibodies and puromycin, further reducing background interference. This method can be performed in fixed cells, allowing researchers to study protein translation in its native cellular context, particularly focusing on specific subcellular locations, such as axons. However, the success of Puro-PLA is contingent upon the availability of high-quality antibodies that specifically recognize the target proteins. Additionally, while the technique demonstrates high specificity, there remains a risk of nonspecific interactions, which could lead to false positives if the assay is not properly optimized. The use of negative controls is an effective strategy to minimize this risk. Puro-PLA has been used to study activity-dependent local translation in dendrites and local synthesis of presynaptic proteins like Bassoon in axons [10]. We used this technique to demonstrate that impaired transport of mRNAs into the axon leads to reduced protein translation and axonal dystrophy [12]. Here, we describe a step-by-step protocol for the detection of RPS7 protein translation in the axons of wildtype (WT) and BORCS5 knock-out (KO) human induced pluripotent stem cell (iPSC)-derived neurons. This protocol can be adapted for the detection of other targets in different types of neurons.
Materials and reagents
Biological materials
1. Human induced pluripotent stem cells (iPSCs) expressing doxycycline-inducible neurogenin 2 (NGN2) [13]
Reagents
1. Essential 8TM Flex Medium kit (E8Flex) (Thermo Fisher Scientific, catalog number: A2858501)
2. Y-27632 dihydrochloride (ROCK inhibitor) (Selleckchem, catalog number: S1049)
3. Accutase (Stem Cell Technologies, catalog number: 07920)
4. Corning Matrigel hESC-qualified matrix, LDEV-free, 5 mL (Matrigel) (Corning, catalog number: 354277)
5. Penicillin/Streptomycin (Quality Biological, catalog number: 120-095-721)
6. DMEM/F12 (Thermo Fisher Scientific, Gibco, catalog number: 11330032)
7. KnockOut DMEM/F12 medium (Thermo Fisher Scientific, Gibco, catalog number: 12660012)
8. MEM non-essential amino acids solution (100×) (MEM-NEAA) (Thermo Fisher Scientific, Gibco, catalog number: 11140050)
9. Non-essential amino acids (NEAA) (Thermo Fisher Scientific, Gibco, catalog number: 11140050)
10. GlutaMAX (Thermo Fisher Scientific, Gibco, catalog number: 35050061)
11. N2A supplement (Thermo Fisher Scientific, Gibco, catalog number: 17502048)
12. Doxycycline (Millipore Sigma, catalog number: D9891)
13. BrainPhys medium (Stem Cell Technology, catalog number: 5790)
14. Human/Mouse/Rat BDNF recombinant protein (BDNF) (PeproTech, Thermo Fisher Scientific, catalog number: 450-02)
15. Human NT-3 recombinant protein (NT-3) (PeproTech, Thermo Fisher Scientific, catalog number: 450-03)
16. B-27TM supplement, serum-free (Thermo Fisher Scientific, Gibco, catalog number: 17504044)
17. Laminin mouse protein, natural (Thermo Fisher Scientific, Gibco, catalog number: 23017015)
18. Poly-L-lysine hydrobromide (Millipore Sigma, catalog number: P2636)
19. Laminin (Roche, Millipore Sigma, catalog number: 11243217001)
20. Paraformaldehyde (PFA) 32% aqueous solution EM grade (Electron Microscopy Sciences, catalog number: 15714-S)
21. Saponin from Quillaja sp. (Millipore Sigma, catalog number: S4521)
22. Bovine serum albumin (BSA), fraction V, fatty acid-free for tissue culture (Gold Biotechnology, catalog number: A-421-250)
23. DAPI Fluoromount-G (Electron Microscopy Sciences, catalog number: 17984-24)
24. Duolink® In Situ Detection Reagents Red kit (Sigma-Aldrich, catalog number: DUO92008)
25. Duolink® blocking solution (Sigma-Aldrich, catalog number: DUO82007)
26. Duolink® in situ PLA probe anti-rabbit PLUS (Sigma-Aldrich, catalog number: DUO92002)
27. Duolink® in situ PLA probe anti-mouse MINUS (Sigma-Aldrich, catalog number: DUO92004)
28. DNA ligase (Sigma-Aldrich, catalog number: DUO82027)
29. Ligation buffer (Sigma-Aldrich, catalog number: DUO82009)
30. DNA polymerase (Sigma-Aldrich, catalog number: DUO82028)
31. Amplification buffer (Sigma-Aldrich, catalog number: DUO82011)
32. Chicken anti-MAP2 antibody (Abcam, catalog number: ab5392)
33. Mouse anti-puromycin antibody, clone 12D10 (Millipore Sigma, catalog number: MABE343)
34. Rabbit anti-RPS7 antibody (MyBioSource, catalog number: MBS9405134)
35. Alexa Fluor 647-conjugated goat anti-chicken IgY (Thermo Fisher Scientific, catalog number: A-21449)
36. Puromycin dihydrochloride (puromycin) (Millipore Sigma, catalog number: P9620-10ML)
37. Water for injection (WFI H2O) for cell culture (Fisher Scientific, Gibco, catalog number: A1287301)
38. Corning 10× phosphate-buffered saline (PBS) (Corning, catalog number: 46-013-CM)
39. Sodium tetraborate decahydrate (Millipore Sigma, catalog number: S9640)
40. Boric acid (Millipore Sigma, catalog number: B6768)
41. HEPES (1 M) (Thermo Fisher Scientific, Gibco, catalog number: 15630080)
42. HBSS (10×) (Thermo Fisher Scientific, Gibco, catalog number: 14185052)
Solutions
1. 1× PBS (see Recipes)
2. Fixative (4% PFA) (see Recipes)
3. Borate buffer pH 8.5 (see Recipes)
4. Poly-L-lysine hydrobromide coating solution) (see Recipes)
5. HANKS solution (see Recipes)
6. Laminin coating solution (see Recipes)
7. BSA solution (see Recipes)
8. BDNF 1,000× (see Recipes)
9. NT-3 1,000× (see Recipes)
10. Doxycycline 1,000× solution (see Recipes)
11. Immunofluorescence blocking buffer (see Recipes)
12. Complete BrainPhys (see Recipes)
13. Induction medium (see Recipes)
14. Complete induction medium (see Recipes)
15. E8 Flex medium (see Recipes)
16. Complete E8 Flex medium (see Recipes)
Recipes
1. 1× PBS
| Reagent | Final concentration | Amount |
|---|---|---|
| 10× PBS | 1× | 100 mL |
| WFI H2O | n/a | 900 mL |
| Total | n/a | 1,000 mL |
2. Fixative (4% PFA)
| Reagent | Final concentration | Amount |
|---|---|---|
| Paraformaldehyde 32% | 4% | 6.25 mL |
| 1× PBS | n/a | 43.75 mL |
| Total | n/a | 50 mL |
3. Borate buffer pH 8.5
| Reagent | Final concentration | Amount |
|---|---|---|
| Sodium tetraborate decahydrate | 100 mM | 38.14 g |
| Boric acid | 100 mM | 6.18 g |
| WFI H2O | n/a | To 1,000 mL |
| HCl | n/a | To pH of 8.5 |
| Total | n/a | 1,000 mL |
Note: Add the sodium tetraborate decahydrate to 800 mL of water and dissolve using a magnetic stirrer at high speed on a heated plate at 37 °C. Once dissolved, add the boric acid and continue stirring until dissolved. Bring the pH to 8.5 with HCl. Use a graduated cylinder to bring the volume to 1,000 mL and filter-sterilize the solution with a 0.22 µm filter size. Do not autoclave, as precipitates may form.
4. Poly-L-lysine hydrobromide coating solution
| Reagent | Final concentration | Amount |
|---|---|---|
| Poly-L-lysine hydrobromide | 0.25 mg/mL | 100 mg |
| Borate buffer | n/a | To 400 mL |
| Total | n/a | 400 mL |
Note: Dissolve the Poly-L-lysine hydrobromide in sterile borate buffer under sterile conditions. Do not filter this solution, as filtering may reduce the Poly-L-lysine hydrobromide concentration.
5. HANKS solution
| Reagent | Final concentration | Amount |
|---|---|---|
| HEPES (1 M) | 10 mM | 5 mL |
| HBSS (10×) | 1× | 50 mL |
| WFI H2O | n/a | 445 mL |
| Total | n/a | 500 mL |
Note: Once mixed, filter-sterilize the solution with a 0.22 µm filter size. Do not autoclave as precipitates may form.
6. Laminin coating solution
| Reagent | Final concentration | Amount |
|---|---|---|
| Laminin | 10 µg/mL | 1 mg |
| HANKS solution | n/a | To 100 mL |
| Total | n/a | 100 mL |
Note: Dissolve the laminin in sterile HANKS solution under sterile conditions. Do not filter this solution, as filtering may reduce the laminin concentration.
7. BSA solution
| Reagent | Final concentration | Amount |
|---|---|---|
| BSA | 0.1% | 50 mg |
| 1× PBS | n/a | To 50 mL |
| Total | n/a | 50 mL |
Note: Once mixed, filter-sterilize the solution with a 0.22 µm filter.
8. BDNF 1,000×
| Reagent | Final concentration | Amount |
|---|---|---|
| BDNF | 10 µg/mL | 10 µg |
| BSA solution | n/a | To 1 mL |
| Total | n/a | 1 mL |
9. NT-3 1,000×
| Reagent | Final concentration | Amount |
|---|---|---|
| NT-3 | 10 µg/mL | 10 µg |
| BSA solution | n/a | To 1 mL |
| Total | n/a | 1 mL |
10. Doxycycline 1,000× solution
| Reagent | Final concentration | Amount |
|---|---|---|
| Doxycycline | 2 mg/mL | 20 mg |
| 1× PBS | n/a | To 10 mL |
| Total | n/a | 10 mL |
11. Immunofluorescence blocking buffer
| Reagent | Final concentration | Amount |
|---|---|---|
| Saponin | 0.1% | 0.1 g |
| BSA reagent | 1% | 1 g |
| 1× PBS | n/a | To 100 mL |
| Total | n/a | 100 mL |
12. Complete BrainPhys
Supplement 50 mL of BrainPhys medium with 50 µL of BDNF 1,000×, 50 µL of NT-3 1,000×, 50 µL of laminin mouse protein, 1 mL of B-27, 500 µL of Penicillin/Streptomycin, and 50 µL of doxycycline 1,000× solution. Store the medium bottle at 4 °C.
13. Induction medium
Supplement 500 mL of DMEM/F12 (1:1) with 5 mL of MEM-NEAA, 5 mL of N2A supplement, 5 mL of GlutaMAX, and 5 mL of Penicillin/Streptomycin. Store the medium bottle at 4 °C.
14. Complete induction medium
Supplement 50 mL of induction medium with 50 µL of ROCK inhibitor and 50 µL of doxycycline. Store the medium at 4 °C for up to one week.
15. E8 Flex medium
Supplement 500 mL of E8Flex with 5 mL of Penicillin/Streptomycin and 5 mL of E8Flex supplement (50×). Store the medium bottle at 4 °C.
16. Complete E8 Flex medium
Supplement 50 mL of E8 Flex medium with 50 µL of ROCK inhibitor. Store the medium at 4 °C for up to one week.
Laboratory supplies
1. 12-mm circular cover glass (Electron Microscopy Sciences, catalog number: 72230-01)
2. Glass slides (Daigger, catalog number: EF15975B)
3. BD Falcon 15 mL centrifuge tube
4. Falcon 50 mL centrifuge tube (Corning, catalog number: 352098)
5. Falcon 15 mL centrifuge tube (Corning, catalog number: 352097)
6. Falcon 24-well clear flat bottom cell culture plate (Corning, catalog number: 253047)
7. Falcon 100 mm TC-treated cell culture dish (Corning, catalog number: 353003)
8. Bemis Parafilm M PM996 (Fisher Scientific, catalog number: 13-374-10)
Equipment
1. LSM 880 inverted confocal microscope with Plan-Apochromat 63× objective (NA = 1.4) (Carl Zeiss)
2. CO2 incubator (Thermo Scientific, model: 3310)
3. Water bath (Thermo Scientific, Labline water bath, model: 2345)
4. Centrifuge (Eppendorf, model: 5702)
Software and datasets
1. Fiji (https://fiji.sc/). The software is free to use.
2. BioRender (https://www.biorender.com/). The following figures were created using BioRender: Graphical overview: BioRender.com/k71x377; Figures 1–3: BioRender.com/x11i230
Procedure
文章信息
稿件历史记录
提交日期: Nov 22, 2024
接收日期: Jan 17, 2025
在线发布日期: Feb 11, 2025
出版日期: Mar 5, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
De Pace, R., Bonifacino, J. S. and Ghosh, S. (2025). Puromycin Proximity Ligation Assay (Puro-PLA) to Assess Local Translation in Axons From Human Neurons. Bio-protocol 15(5): e5224. DOI: 10.21769/BioProtoc.5224.
分类
神经科学 > 细胞机理
细胞生物学 > 细胞成像 > 共聚焦显微镜
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link