- EN - English
- CN - 中文
Visualization of Gap Junction–Mediated Astrocyte Coupling in Acute Mouse Brain Slices
急性小鼠大脑切片中缝隙连接介导的星形胶质细胞耦合可视化
发布: 2025年02月20日第15卷第4期 DOI: 10.21769/BioProtoc.5220 浏览次数: 2255
评审: Anonymous reviewer(s)

相关实验方案
采用 Davidson 固定液和黑色素漂白法优化小鼠眼组织切片的免疫组化染色
Anne Nathalie Longakit [...] Catherine D. Van Raamsdonk
2025年11月20日 1562 阅读
Abstract
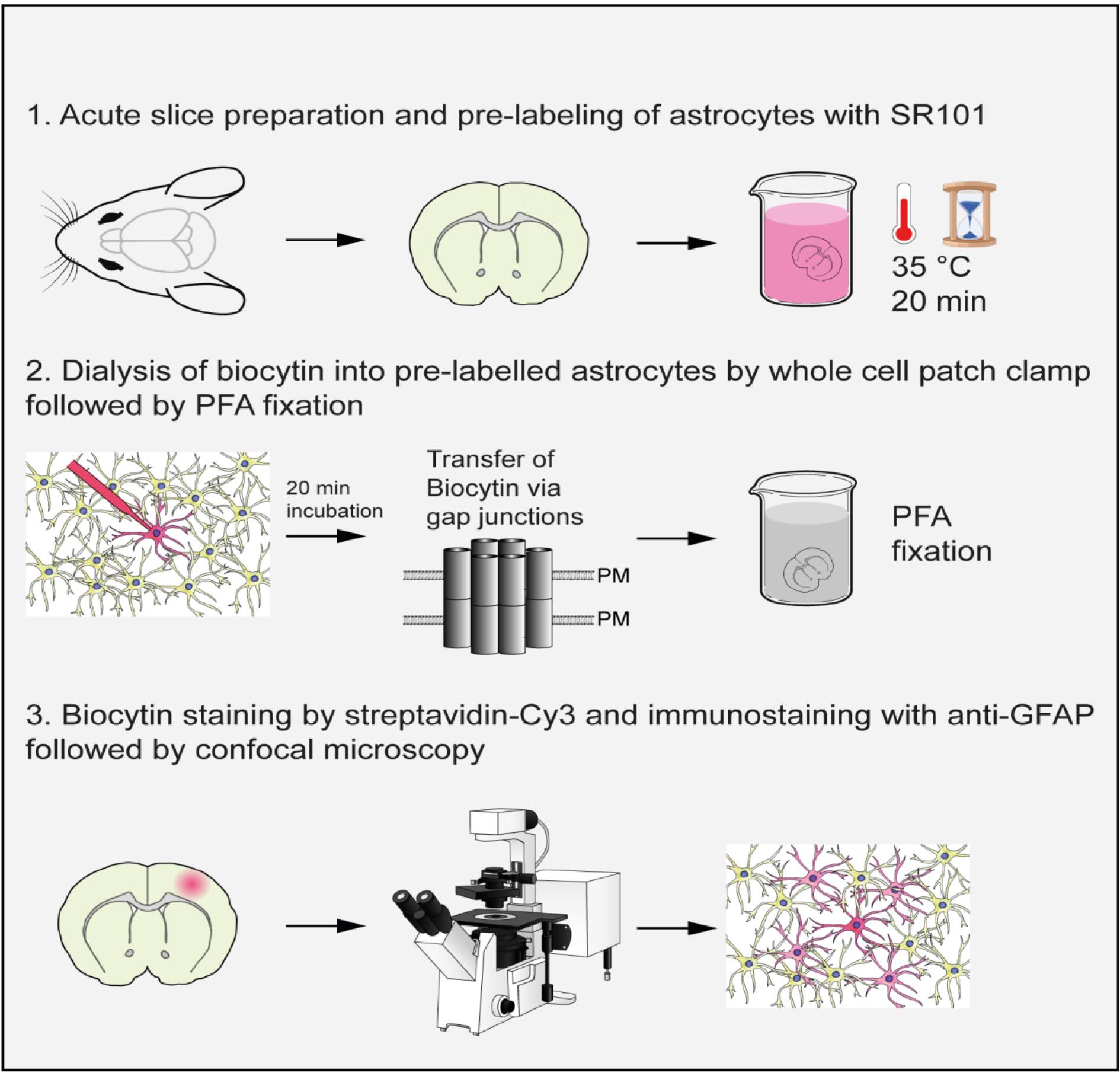
Gap junctions are transmembrane protein channels that enable the exchange of small molecules such as ions, second messengers, and metabolites between adjacent cells. Gap junctions are found in various mammalian organs, including skin, endothelium, liver, pancreas, muscle, and central nervous system (CNS). In the CNS, they mediate coupling between neural cells including glial cells, and the resulting panglial networks are vital for brain homeostasis. Tracers of sufficiently small molecular mass can diffuse across gap junctions and are used to visualize the extent of cell-to-cell coupling in situ by delivering them to a single cell through sharp electrodes or patch-clamp micropipettes. Here, we describe a protocol for pre-labeling and identification of astrocytes in acute mouse forebrain slices using Sulforhodamine 101 (SR101). Fluorescent cells can then be targeted for whole-cell patch-clamp, which allows for further confirmation of astroglial identity by assessing their electrophysiological properties, as well as for passive dialysis with a tracer such as biocytin. Slices can then be subjected to chemical fixation and immunostaining to detect dye-coupled networks. This protocol provides a method for the identification of astrocytes in live tissue through SR101 labeling. Alternatively, transgenic reporter mice can also be used to identify astrocytes. While we illustrate the use of this protocol for the study of glial networks in the mouse brain, the general principles are applicable to other species, tissues, and cell types.
Key features
• Pre-labeling of live astrocytes in acute adult mouse brain slices using the dye Sulforhodamine 101.
• Dialysis of biocytin into individual astrocytes using whole-cell patch-clamp electrophysiology.
• Staining of biocytin by streptavidin and immunostaining of GFAP, imaging, and analysis of dye-coupled astrocytic networks.
• Can be used for other glial cell types and might be adapted to other tissues and species.
Keywords: Astrocytes (星形胶质细胞)Graphical overview
Background
Gap junctions are transmembrane protein channels that allow for the exchange of ions and small molecules, including second messengers and metabolites, between adjacent cells [1–4]. These channels are formed by a protein family called connexins that is found in various mammalian organs, including the skin, endothelium, liver, pancreas, muscle, and central nervous system (CNS) [5–7]. In the CNS, gap junctions facilitate communication between neural cells, particularly astrocytes that form large networks. Gap junction coupling between identified astrocytes has first been described in primary mouse cultures. After injection of the gap junction–permeable dye Lucifer Yellow into one astrocyte, the dye spread into the network of adjacent cells. After subsequent labeling of the cells with the astrocyte-specific marker glial fibrillary acidic protein (GFAP), it was evident that the dye had spread within the astrocyte cell population [8]. The first evidence for coupling between identified glial cells in an acute slice preparation was obtained in the cerebellum [9]. Interastrocytic, interoligodendrocytic, and astro-oligodendrocytic coupling has been shown in situ in both grey and white matter by dye-coupling experiments through dialysis of a gap-junction permeable dye or tracer into one cell through sharp electrodes or patch-clamp pipettes [10–13]. By contrast, microglia are not incorporated into gap-junction coupled networks [14,15]. The size, shape, and composition of coupled networks are highly variable depending on the region analyzed [12,13,16,17] and are altered in pathological conditions, for example in mouse models of Alzheimer’s disease [18] and temporal lobe epilepsy [19,20].
In situ identification of cells for dye-coupling experiments often relies on transgenic reporter mice expressing fluorescent proteins under cell type–specific promoters to identify cells of interest [12,13,16,17]. However, these lines can be expensive to maintain or have to be crossbred if studies require other genetic manipulations. Moreover, some reporter lines show ectopic expression, such as astrocyte reporter lines labeling NG2 glia in some regions [17,21,22].
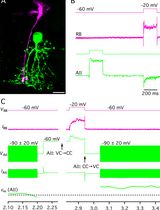
Sulforhodamine 101 is a non-toxic dye that is preferentially taken up by astrocytes upon topical application [23,24]. It is therefore a quick and inexpensive alternative to identify astrocytes and can be applied in any mouse model of interest. Using a patch-clamp rather than sharp electrodes to passively dialyze SR101-positive cells with a gap junction–permeable dye or tracer such as biocytin, a simple voltage step protocol can be performed to confirm astrocyte identity. In addition, the patch-clamp allows the researcher to monitor cellular health and the quality of the dialysis process. Post-hoc staining and imaging of biocytin diffusion allows for the reliable detection and characterization of dye-coupled cells. Different tracers of different molecular masses and charges, such as Lucifer Yellow (457.25 Da; charge -2 at neutral pH), ethidium bromide (394.32 Da; charge +1), and biocytin (372.47; net charge: 0) have different diffusion rates [25,26], which should be taken into account to allow for comparison between studies. Biocytin, the tracer used in this protocol, is a derivative of biotin. It has strong affinity for avidin or streptavidin and can therefore easily be visualized in brain slices. Neurobiotin, another biotin derivative, has a higher solubility but a net charge of + 1 and thus a different diffusion rate than biocytin.
Materials and reagents
Reagents
1. Sucrose (Sigma-Aldrich, catalog number: 4621.2)
2. NaHCO3 (Sigma-Aldrich, catalog number: 6885.1)
3. KCl (Sigma-Aldrich, catalog number: 6781.1)
4. NaH2PO4·H2O (AppliChem, catalog number: 1319651211)
5. MgSO4 (Sigma-Aldrich, catalog number: 8283.2)
6. CaCl2 (Sigma-Aldrich, catalog number: A119.1)
7. Glucose (Sigma-Aldrich, catalog number: 6887.1)
8. NaCl (Sigma-Aldrich, catalog number: 3957.2)
9. MgCl2 (Sigma-Aldrich, catalog number: KK36.1)
10. K2HPO4·3H2O (Sigma Merck, catalog number: 1.05099.100)
11. Sulforhodamine 101 (Sigma-Aldrich, catalog number: MKBJ0694/MKCD2256)
12. EGTA (Sigma-Aldrich, catalog number: E0396-100G)
13. HEPES (Carl Roth, catalog number: 9105.3)
14. Na2ATP (Sigma Merck, catalog number: A7699)
15. Potassium gluconate (Sigma-Aldrich, catalog number: B4261)
16. Biocytin (Sigma-Aldrich, catalog number: 576-19-2)
17. Lucifer Yellow (Sigma-Aldrich, catalog number: L0259-100MG)
18. Paraformaldehyde (Sigma-Aldrich, catalog number: P6148-1KG)
19. Triton X-100 (Carl Roth, catalog number: 3051.3)
20. Bovine serum albumin (Carl Roth, catalog number: 0163.2)
21. Normal donkey serum (Sigma Merck, catalog number: S30-100ml)
22. Tween 20 (Carl Roth, catalog number: 9127.1)
23. DAPI (Sigma-Aldrich, catalog number: D9542)
24. Cy3-conjugated streptavidin (Dianova, catalog number: 016-160-084)
25. Guinea-pig anti-GFAP antibody (Synaptic systems, catalog number: 173004)
26. Alexa Fluor 488-conjugated donkey anti-rabbit IgG (Dianova, catalog number: 711-545-152)
27. Alexa Fluor 647-conjugated donkey anti-guinea pig IgG (Dianova, catalog number: 706-605-148)
28. Phosphate-buffered saline (PBS) (Sigma-Aldrich, catalog number: P5244)
29. Tris-buffered saline (TBS) (Sigma-Aldrich, catalog number: T5912
Solutions
1. Slicing solution (see Recipes)
2. Artificial cerebrospinal fluid (ACSF) (see Recipes)
3. ACSF with Sulforhodamine 101 (see Recipes)
4. Intracellular solution (ICS) with 0.5% biocytin (see Recipes)
5. Fixation solution with Paraformaldehyde (PFA) (see Recipes)
6. Blocking solution for immunohistochemistry (see Recipes)
7. Antibody incubation solution for immunohistochemistry (see Recipes)
8. Washing solution for immunohistochemistry (see Recipes)
Recipes
1. Slicing solution
Note: Slicing solution should be maintained at 4 °C during immediate use and continuously carbonated (95% O2, 5% CO2). The desired final volume is dependent on the size of the slicing chamber and could therefore differ from 500 mL.
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Sucrose | 230 mM | 39.3645 g |
| NaHCO3 | 26 mM | 1.09213 g |
| KCl | 2.5 mM | 0.0932 g |
| NaH2PO4 | 1.25 mM | 0.0862 g |
| MgSO4 | 10 mM | 1.2324 g |
| CaCl2 | 0.5 mM | 0.02775 g |
Glucose dH2O | 10 mM n/a | 0.99085 g Q.S. 500 mL |
| Total | n/a | mL |
2. ACSF
Note: ACSF should be used at room temperature and continuously carbonated (95% O2, 5% CO2). It can be kept for one week at 4 °C.
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| NaCl | 134 mM | 7.83096 g |
| KCl | 2.5 mM | 0.1864 g |
| MgCl2 | 1.3 mM | 0.1238 g |
| CaCl2 | 2 mM | 0.22198 g |
| K2HPO4 | 1.26 mM | 0.2195 g |
| NaHCO3 | 26 mM | 2.1842 g |
| Glucose | 10 mM | 1.9817 g |
| dH2O | n/a | Q.S. 1 L |
| Total | n/a | 1 L |
3. ACSF with Sulforhodamine 101
Note: ACSF with Sulforhodamine 101 should be used at 35 °C and continuously carbonated (95% O2, 5% CO2). Can be aliquoted into 250 mL aliquots, stored at -20 °C, and freshly thawed for each experiment.
| Reagent | Final concentration | Quantity or Volume | |
|---|---|---|---|
| NaCl | 134 mM | 7.83096 g | |
| KCl | 2.5 mM | 0.1864 g | |
| MgCl2 | 1.3 mM | 0.1238 g | |
| CaCl2 | 2 mM | 0.22198 g | |
| K2HPO4 | 1.26 mM | 0.2195 g | |
| NaHCO3 | 26 mM | 2.1842 g | |
| Glucose | 10 mM | 1.9817 g | |
| dH2O | n/a | Q.S. 1 L | |
| Sulforhodamine 101 | 1 μM | 0.0006 g | |
| Total | n/a | 1 L |
4. ICS solution with 0.5% biocytin
Note: 100 mL of ICS solution can be prepared ahead of time; 1 mL aliquots should be kept frozen at -20 °C. For each experiment, thaw 1 mL of ICS solution and add fresh 0.5% biocytin (0.005 mg) and Lucifer Yellow (10 μg/mL). (Note that Lucifer Yellow is added to the patch-pipette to monitor the outflow.) Before each experiment, filter the ICS solution using a 0.22 μm filter unit (see laboratory supplies).
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| KCl | 30 mM | 0.22368 g |
| EGTA | 5 mM | 0.19018 g |
| HEPES | 10 mM | 0.23831 g |
| MgCl2 | 1 mM | 0.00952 g |
| Na2ATP | 3 mM | 0.16534 g |
| CaCl2 | 0.5 mM | 0.00555 g |
| Potassium gluconate | 100 mM | 2.34246 g |
| dH2O | n/a | Q.S. 100 mL |
| Total | n/a | 100 mL |
5. Fixation solution with paraformaldehyde
Note: After adding paraformaldehyde to the PBS, slowly adjust the pH to 7.4 by adding NaOH dropwise. Aliquot and store at -20 °C.
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Paraformaldehyde | 40 g/L | 40 g |
| Phosphate buffered saline | n/a | Q.S. 1 L |
| Total | n/a | 1 L |
6. Blocking solution for immunohistochemistry
Note: Store at -20 °C
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Triton X-100 | 2 mL/100 mL | 2 mL |
| Bovine serum albumin | 2 mg/100 mL | 2 mg |
| Normal donkey serum | 5 mL/100 mL | 5 mL |
| Tris-buffered saline | n/a | Q.S. 100 mL |
| Total | n/a | 100 mL |
7. Antibody incubation solution for immunohistochemistry
Note: Store at -20 °C.
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Triton X-100 | 1 mL/100 mL | 1 mL |
| Bovine serum albumin | 1 mg/100 mL | 1 mg |
| Normal donkey serum | 2.5 mg/100 mL | 2.5 mg |
| Tris-buffered saline | n/a | Q.S. 100 mL |
| Total | n/a | 100 mL |
8. Washing solution for immunohistochemistry
Note: Store at -20 °C.
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Bovine serum albumin | 1 mg/100 mL | 1 mg |
| Tween 20 | 0.1 mL/100 mL | 0.1 mL |
| Tris-buffered saline | n/a | Q.S. 100 mL |
| Total | n/a | 100 mL |
Laboratory supplies
1. 2.5 mL SafeSeal reaction tubes (Sarstedt, catalog number: 72.695.400)
2. 1.5 mL Eppendorf tubes (Sarstedt, catalog number: 72.706.400)
3. Millex 0.22 μm filter unit (Merck Millipore, catalog number: SLGV004SL)
4. 24-well plates (Sarstedt, catalog number: 83.3922)
5. 400 mL glass beaker (fitted with six transparent PET cell culture inserts with nylon stockings glued to the bottom to serve as “chambers” for brain slices) (Carl Roth, catalog number: X692.1)
6. Transparent PET cell culture inserts (Falcon, catalog number: 353090)
7. 100 mL glass beaker (fitted with one transparent PET cell culture insert with nylon stockings glued to the bottom to serve as “chambers” for brain slices) (Carl Roth, catalog number: X689.1)
8. X10 double-sided razor blades (for vibratome) (Fisher Scientific, catalog number: 12043029)
9. Decapitation scissors (Fine Science Tools, catalog number: 14200-21)
10. Standard dissection kit (Deutsche Biomedical, catalog number: DBK1016)
11. Pasteur pipettes (Carl Roth, catalog number: EA70.1)
12. Filter paper (Carl Roth, catalog number: L875.1)
13. Tygon standard hoses S3 ID 1.6 mm × OD 3.2 mm (Carl Roth, catalog number: E-3606)
14. Glass Petri dish 10 cm Ø (Medplus catalog number: 54693)
15. Reusable blades (Carl Roth, catalog number: CK07.1)
16. Superglue (“Sekundenkleber” from UHU)
17. 20 μL micro loader pipette tips (Eppendorf, catalog number: 5242 956.003)
18. 1 mL syringe Braun Omnifix F Luer Solo (B Braun, catalog number: 9161406V)
19. Platinum harp slice anchor, custom-made in-house, but equivalents can be purchased from e.g., Warner instruments or NPI electronic
20. Borosilicate glass, 1.5 mm outside diameter, 0.315 mm wall thickness (Hilgenberg, catalog number: 1409249)
21. 15 mL centrifuge tubes (Biozym, catalog number: LC0052)
22. Glass Pasteur pipettes, length 150 mm (Fisher Scientific, catalog number: 1154-6963)
23. Disposable needles Terumo 0.9 × 38 mm (CLS Medizintechnik, catalog number: AN*2038R1)
24. Brush (TH.Geyer, catalog number: D770000)
25. Superfrost plus 25 × 75 × 1.0 mm glass slides (Epredia, catalog number: AB000008032ED1MNZ50)
26. Absorbing tissue, KIMTECH, Science Precision tissue, 7552, 286 pieces (Carl Roth, catalog number: AA64.1)
27. Cryotags (Carl Roth, catalog number: X547.1)
28. Coverslips, 24 × 40 mm (Carl Roth, catalog number: 1870)
29. Slide folder (Carl Roth, catalog number: AXK6.1)
30. Aqua Polymount mounting medium (Polysciences, catalog number: 18606-5)
Equipment
1. Vibratome (Microm International GmbH, model: HM 650V)
2. Osmometer (Gonotec GmbH, model: Osmomat 3000 basic)
3. Incubator (Melag Medizintechnik, model: Type A)
4. Pipette puller (Sutter Instrument, model: P-97)
5. Anti-vibration air table (Microplan Schwingungstechnik)
6. Microscope (Slicescope II, Scientifica Ltd., or Axioskop 2 FS Plus, Carl Zeiss Microscopy GmbH)
7. CCD camera for microscope (Imago SensiCam, model: PCO AG)
8. Fluorescent light source VSG HBO 100/001.26E (JENA GmbH, catalog number: 2029)
9. Peristaltic pump for ACSF superfusion (Minipuls 3, AbiMed Gilson, Inc. or Periflo Flocon 1003, PetroGas Ausrüstungen Berlin GmbH)
10. 5× objective (Olympus, model: MPLFLN)
11. 60× water immersion objective (Olympus, catalog number: 1-U2B893)
12. Monochromator (Polychrome IV, Till Photonics GmbH, catalog number: 5855)
13. Filter set for SR101 (excitation and emission wavelengths of 555 and 585 ± 10 nm) (Leica)
14. Filter set multi-band XF53 (Omega Optical)
15. EPC 10 patch-clamp amplifier (HEKA Elektronik, catalog number: 895277)
16. Micromanipulator (Patch Star micromanipulator, Scientifica)
17. Patch-clamp control panels (Patch Star, Scientifica)
18. Stuart gyro-rocker SSL3 (Sigma-Aldrich, catalog number: Z654515)
19. Centrifuge (Eppendorf, model: 5417R)
20. Leica DM TCS SPE confocal microscope (Leica Microsystems GmbH, model: HC APO 20×/0.75)
Software and datasets
1. TIDA 5.25 (HEKA Elektronik); license required
2. Igor Pro 7 (WaveMetrics); license required
3. LCS Lite or LAS AF Lite (Leica); license required
4. Fiji/ImageJ (NIH); free to use
5. Prism 6.07 and 10.1.2 (GraphPad); license required
6. CamWare for HSFC-pro 3.07 (PCO AG); license required
Procedure
文章信息
稿件历史记录
提交日期: Oct 1, 2024
接收日期: Dec 12, 2024
在线发布日期: Feb 6, 2025
出版日期: Feb 20, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/).
如何引用
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Kompier, N. F., Siemonsmeier, G., Meyer, N., Kettenmann, H. and Rathjen, F. G. (2025). Visualization of Gap Junction–Mediated Astrocyte Coupling in Acute Mouse Brain Slices. Bio-protocol 15(4): e5220. DOI: 10.21769/BioProtoc.5220.
- Pelz, L., Dossou, L., Kompier, N., Jüttner, R., Siemonsmeier, G., Meyer, N., Lowenstein, E. D., Lahmann, I., Kettenmann, H., Birchmeier, C., et al. (2024). The IgCAM BT-IgSF (IgSF11) Is Essential for Connexin43-Mediated Astrocyte–Astrocyte Coupling in Mice. eNeuro. 11(3): ENEURO.0283–23.2024. https://doi.org/10.1523/eneuro.0283-23.2024
分类
神经科学 > 细胞机理
细胞生物学 > 细胞染色 > 全细胞
生物物理学 > 电生理 > 膜片钳技术
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link